A Case Study of Yankari Game Reserve, Nigeria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conservation Strategies of Wildlife Resources in the Old Oyo National Park, Nigeria
International Journal of Advanced Scientific and Technical Research Issue 4 volume 4, July-August 2014 Available online on http://www.rspublication.com/ijst/index.html ISSN 2249-9954 CONSERVATION STRATEGIES OF WILDLIFE RESOURCES IN THE OLD OYO NATIONAL PARK, NIGERIA. 1 2 3 Toyobo, Adigun E. Raheem, Wasiu M. Oyeleye, Oyewale I. 1,2&3Department of Urban and Regional Planning, Faculty of Environmental Sciences, Ladoke Akintola University of Technology, P.M.B 4000, Ogbomoso, Oyo State, Nigeria. E-mails: [email protected] [email protected] [email protected] ABSTRACT Wildlife and plants are major part of nature which goes to a large extent in providing means of livelihood for varying categories of people in the world. Human beings are however engaged in over exploitation of this segment of the ecosystem, so much that many species are becoming endangered. It is against this background that this study evaluates the conservation strategies of wildlife resources in Nigerian national parks with particular reference to the old Oyo National Park. Data used for the study were primary data obtained through random sampling of 60 respondents in Ikoyi-Ile, an adjoining settlement, richer in the fauna population of the park. Data collected were analyzed using descriptive statistic and chi square statistics. The result shows considerable degree of effectiveness of the strategies employed by the authority of the park, as majority of the respondents are aware of the status of the park as a protected area. The paper therefore concludes by emphasizing the need to brace up in the various conservation programmes of the park. -

ISSN: 2149-6528 2016 Vol. 1, Issue.1 SUSTAINABLE WILDLIFE
Journal of Tourism and Management Research 101 ISSN:2149-6528 Journal of Tourism and Management Research ISSN: 2149-6528 2016 Vol. 1, Issue.1 SUSTAINABLE WILDLIFE CONSERVATION AT OKOMU NATIONAL PARK, NIGERIA Abstract The purpose of this study is to investigate the various sustainable preservation measures at Okomu National Park, Benin, Nigeria, identify the various challenges of the Park and suggest the way forward. Ethnography was adopted where research methods like key-informant interview, focus-group discussion, field observation, and electronic documentation were used to elicit data from the field. Relevant documentary sources were not left out either. Data gathered were analysed using descriptive and narrative analytical methods. This revealed a range of management measures that were grouped into two main categories (i.e. Educational measures and Technical measures). Major challenges like poaching and community violence were identified, with the major causes arising from host communities. At the end the study made a useful contribution to the sustainable management of parks for ecotourism development, by introducing the HoPSuP Model as a management practice to encourage a healthy host-park relationship in Nigeria and some other developing nations. Keywords: sustainability, wildlife, conservation, national park, challenges, HoPSuP model. _________________________________ Elochukwu A. Nwankwo, PhD. Department of Archaeology and Tourism / University of Nigeria Nsukka. Email: [email protected] Aishat Halilu, M.A. Department of Archaeology and Tourism / University of Nigeria, Nsukka. Email: [email protected] Original Scientific Paper Nwankwo, E.A and Halilu, A. Vol.1/No.1/2016/101-118. DOI:10.26465/ojtmr.2016132258 Journal of Tourism and Management Research 102 1. -
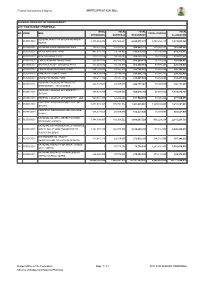
Appropriation Bill
Federal Government of Nigeria APPROPRIATION BILL FEDERAL MINISTRY OF ENVIRONMENT 2017 FGN BUDGET PROPOSAL TOTAL TOTAL TOTAL TOTAL NO CODE MDA TOTAL CAPITAL PERSONNEL OVERHEAD RECURRENT ALLOCATION FEDERAL MINISTRY OF ENVIRONMENT - 1. 0535001001 2,173,269,254 672,562,421 2,845,831,675 4,567,262,147 7,413,093,823 HQTRS 2. 0535002001 NATIONAL PARK HEADQUARTERS 193,634,708 54,447,671 248,082,379 514,004,581 762,086,960 3. 0535003001 KAINJI NATIONAL PARK 482,677,775 53,198,837 535,876,612 82,500,000 618,376,613 4. 0535004001 OYO NATIONAL PARK 389,743,876 50,590,121 440,333,997 55,483,225 495,817,222 5. 0535005001 CHAD BASIN NATIONAL PARK 228,681,496 40,539,137 269,220,633 56,700,000 325,920,633 6. 0535006001 GASHAKA GUMTI NATIONAL PARK 312,443,632 50,105,537 362,549,169 57,970,463 420,519,633 7. 0535007001 CROSS RIVER NATIONAL PARK 482,142,617 50,563,970 532,706,587 93,650,000 626,356,587 8. 0535008001 KAMUKU NATIONAL PARK 194,859,429 38,796,289 233,655,718 60,666,775 294,322,493 9. 0535009001 OKUMU NATIONAL PARK 180,214,108 33,847,716 214,061,824 40,350,000 254,411,824 FEDERAL COLLEGE OF WILDLIFE 10. 0535010001 436,737,061 60,000,000 496,737,061 50,000,000 546,737,061 MANAGEMENT - NEW BUSSA FEDERAL COLLEGE OF FORESTRY - 11. 0535011001 889,578,759 70,000,000 959,578,759 60,000,000 1,019,578,759 IBADAN 12. -

Biodiversity in Sub-Saharan Africa and Its Islands Conservation, Management and Sustainable Use
Biodiversity in Sub-Saharan Africa and its Islands Conservation, Management and Sustainable Use Occasional Papers of the IUCN Species Survival Commission No. 6 IUCN - The World Conservation Union IUCN Species Survival Commission Role of the SSC The Species Survival Commission (SSC) is IUCN's primary source of the 4. To provide advice, information, and expertise to the Secretariat of the scientific and technical information required for the maintenance of biologi- Convention on International Trade in Endangered Species of Wild Fauna cal diversity through the conservation of endangered and vulnerable species and Flora (CITES) and other international agreements affecting conser- of fauna and flora, whilst recommending and promoting measures for their vation of species or biological diversity. conservation, and for the management of other species of conservation con- cern. Its objective is to mobilize action to prevent the extinction of species, 5. To carry out specific tasks on behalf of the Union, including: sub-species and discrete populations of fauna and flora, thereby not only maintaining biological diversity but improving the status of endangered and • coordination of a programme of activities for the conservation of bio- vulnerable species. logical diversity within the framework of the IUCN Conservation Programme. Objectives of the SSC • promotion of the maintenance of biological diversity by monitoring 1. To participate in the further development, promotion and implementation the status of species and populations of conservation concern. of the World Conservation Strategy; to advise on the development of IUCN's Conservation Programme; to support the implementation of the • development and review of conservation action plans and priorities Programme' and to assist in the development, screening, and monitoring for species and their populations. -

TECHNICAL REPORT Issue 1, No
TECHNICAL REPORT Issue 1, No. 1, March, 2019 REPORT ON THE NEED TO RATIONALIZE AND RESTRUCTURE FEDERAL MINISTRIES, DEPARTMENTS AND AGENCIES (MDAs) Edoba B. Omoregie, Tonye C. Jaja, Samuel Oguche and Usman Ibrahim Department of Legislative Support Services Background/Rationale Worried by the non-implementation of the Steve Oronsaye Report recommending the rationalisation and restructuring of Federal Ministries, Departments and Agencies (MDAs), the National Institute for Legislative and Democratic Studies constituted a four-man Technical Committee to study the Oronsaye Report vis-à- vis existing agencies, and make appropriate recommendations on the way forward, including drafting proposed legislations (Bills) in practical response to specific issues raised, where necessary. From a financial, administrative and legal point-of-view, it is now a global standard practice of modern legislatures around the world to undertake Regulatory Impact Assessment (RIA) and Legislative Impact Analysis (LIA), even if it is a “rudimentary cost-benefit analysis”1 of proposed legislation. The primary purpose of such RIA and LIA, or cost-benefit analysis of proposed legislation is to enable legislators make an informed decision based on concrete evidence that a proposed legislation is beneficial for the greater number of citizens. In Nigeria, as evident from the 2011 Steve Oronsaye Report, the failure to undertake RIA and LIA/rudimentary cost-benefit analysis of proposed legislation (especially for those category of legislation that seeks to establish statutory -

National Assembly 2071 2013 Appropriation
FEDERAL GOVERNMENT OF NIGERIA 2012 BUDGET SUMMARY FEDERAL MINISTRY OF ENVIRONMENT TOTAL OVERHEAD CODE TOTAL PERSONNEL TOTAL RECURRENT TOTAL CAPITAL TOTAL ALLOCATION COST MDA COST =N= =N= =N= =N= =N= 0535001001 MAIN MINISTRY 154,077,328 325,153,770 479,231,098 9,257,672,550 9,736,903,648 0535002001 NATIONAL PARK HEADQUARTER 142,697,404 90,335,409 233,032,813 606,060,008 839,092,821 0535003001 KAINJI LAKE NATIONAL PARK 342,889,081 75,235,124 418,124,205 10,000,000 428,124,205 0535004001 OLD OYO NATIONAL PARK 243,239,134 75,804,391 319,043,525 29,500,000 348,543,525 0535005001 CHAD BASIN NATIONAL PARK 202,953,024 77,943,517 280,896,540 19,900,000 300,796,540 0535006001 GASHAKA GUMTI NATIONAL PARK 249,350,902 80,277,492 329,628,394 49,400,000 379,028,394 0535007001 CROSS RIVER NATIONAL PARK 337,685,668 90,184,292 427,869,960 620,000,000 1,047,869,960 0535008001 KUMUKU NATIONAL PARK 142,531,836 76,173,544 218,705,380 35,000,000 253,705,380 0535009001 OKUMU NATIONAL PARK 136,862,654 77,004,109 213,866,763 10,000,000 223,866,763 FEDERAL COLLEGE OF WILD LIFE 0535010001 MANAGEMENT NEW BUSSA 348,377,873 125,089,396 473,467,269 50,000,000 523,467,269 0535011001 FEDERAL COLLEGE OF FORESTRY IBADAN 691,884,226 120,961,152 812,845,378 34,806,203 847,651,581 0535012001 FEDERAL COLLEGE OF FORESTRY, JOS 470,270,647 150,713,191 620,983,838 280,295,507 901,279,345 FORESTRY RESEARCH INSTITUTE OF NIGERIA, 0535013001 IBADAN 1,468,773,710 299,668,324 1,768,442,034 186,510,623 1,954,952,657 0535014001 FORESTRY MECHANISATION COLLEGE, AFAKA 413,536,783 100,558,024 -

Egypt. Acad. J. Biolog. Sci. (B. Zoology) Vol. 11(3)Pp 1-11 (2019) Egypt
Vol. 11 No. 3 (2019) Citation: Egypt. Acad. J. Biolog. Sci. (B. Zoology) Vol. 11(3)pp 1-11 (2019) Egypt. Acad. J. Biolog. Sci., 11(3): 1- 11 (2019) Egyptian Academic Journal of Biological Sciences B. Zoology ISSN: 2090 – 0759 www.eajbsz.journals.ekb.eg Conservation Attitudes and Challenges: A Study of Forest Elephants In Southern Nigeria Forests Queen O. Omoregie1; Bola O. Oboh2; and Rosemary I. Egonmwan1 1. Department of Zoology, University of Lagos, Nigeria. 2. Department of Cell Biology and Genetics, University of Lagos, Nigeria. E.Mail: [email protected] _____________________________________________________________________ ARTICLE INFO ABSTRACT Article History Elephant populations in Southern Nigeria are small and most of Received:2 /10/2019 them exist in forest islands isolated by farms, monoculture plantations, Accepted:1/11/2019 and human habitations. Their close proximity to communities often _________________ results in Human-Elephant Conflicts (HEC). Awareness of, and attitudes Keywords: towards the conservation of elephants in four elephant ranges in Southern Forest elephants, Nigeria were determined using field observations, questionnaires, and conservation interviews. The major threats to the species’ sustainability were attitudes, Human- development in and around the forests (24.1%), logging (22.0%) and Elephant hunting activities (20.0%). Incidences of HEC as a result of crop- Conflicts, raiding/damage, non-payment of compensation to farmers whose crops Southern Nigeria were raided and perceived highhandedness of -

An Assessment of Support Zone Community Participation for Sustainable Development in Kainji Lake National Park, Niger State, Nigeria
Research Journal of Management Practice | ISSN: 2782-7674 Vol. 1, Issue 4 (April, 2021) | www.ijaar.org Journal DOI: 10.46654/RJMP Article DOI: 10.46654/RJMP.1402 AN ASSESSMENT OF SUPPORT ZONE COMMUNITY PARTICIPATION FOR SUSTAINABLE DEVELOPMENT IN KAINJI LAKE NATIONAL PARK, NIGER STATE, NIGERIA ADENIYI G .T. (MRS) DEPARTMENT OF LEISURE AND TOURISM MANAGEMENT, FEDERAL POLYTECHNIC IDAH, KOGI STATE. TEL: 07036072187 Email: [email protected] ABSTRACT The paper examined the support zone community for sustainable development in Kainji lake national park Niger State. Ten (10) communities were randomly selected and the national park service staff. Five (5) communities from each sector of the support zone communities surrounding the National park with a total population of three hundred (300) respondents. The study adopted structured questionnaire method to collect data. Three hundred (300) structured questionnaires were distributed to respondents and two hundred and thirty eight [238] questionnaires were completed and returned and used for the study. Data collected were analyzed using Chi-square analysis. Result revealed that there are certain reactions shown by the people of the communities for the government to have taken their land without compensation n= 238, p(0.000) <0.05. The study further shows that Kainji Lake National Park has contributed positively to the growth and development of the communities especially to those communities around the national park and the park has contributed economically in the area of job creation, infrastructure, agricultural support and supplies of high breed seed, provision of loan for small scale business, improvement in educational system. The paper recommends that, the support zone communities should be encouraged the more to actively participate in wildlife conservation management for sustainable development. -
Elixir Journal
53476 Jones M. Jaja/ Elixir Social Studies 132 (2019) 53476-53485 Available online at www.elixirpublishers.com (Elixir International Journal) Social Studies Elixir Social Studies 132 (2019) 53476-53485 Harnessing Tourism Potentials of Nigeria: The Opportunities in the Niger Delta Region Jones M. Jaja1, Emmanuel Obuah2 and Joy Agumagu3 1Department of History and International Diplomacy, Rivers State University, Port Harcourt, Rivers State 2Department of History and Diplomatic Studies, University of Port Harcourt, Rivers State 3Department of Educational Foundations, Rivers State University, Port Harcourt, Rivers State ARTICLE INFO ABSTRACT Article history: The important role that the tourism sector plays as the main instrument in the socio- Received: 4 June 2019; economic development of any nation cannot be overemphasized. The nation of Nigeria is Received in revised form: a multi-religious and multi-ethnic state with over two hundred and fifty ethnic groups 20 July 2019; that are rich in diverse cultures. Nature has so blessed the country with vast land, Accepted: 31 July 2019; beautiful climate and land formation, especially with the swampy coastal lands and beaches to the lush rain forests and the savannah. There are also the parks and game Keywords reserves in their natural habitat. The hills, the rocks, lakes, waterfalls, natural and warm Assets, springs serve to make the landscape so beautiful. This paper seeks to highlight the place Tourism, of tourism in employment generation together with a succinct outline of tourism Economy, institutional evolution in the Nigerian context. There are so many natural endowments Employment, that are yet to be harnessed in full capacity and requiring attention (of the tourism Development, industry) for maximum exploitation and benefits which, no doubt, will increase the Culture, economic fortune of the country. -
National Open University of Nigeria School of Science and Technology Course Code: Bio 412 Course Title: Wildlife Ecology And
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 412 COURSE TITLE: WILDLIFE ECOLOGY AND CONSERVATION 1 BIO 412 WILDLIFE ECOLOGY AND CONSERVATION (3 UNITS) Module 1 Unit 1 1.0 Introduction: General principles of ecosystems management 2.0 Objectives 3.0 Main content 3.1. Principles of sustainable management of the ecosystems 3.2 Ecosystems 3.3 Tropical Rainforest 3.3.1 Forest plants 3.3.2 Animal that lives in the rainforest 4.0 Conclusion 5.0 Summary 6.0 Tutor Marked Assignment (TMA) 7.0 References/Further reading. Module 1 Unit 1: General principles of ecosystem Management 1.0 Introduction: The aim of wildlife management is to maintain and improve wildlife and control animal popula- tion by managing the habitat. The management is concerned primarily with production of quality 2 and quantity on a sustained yield basis, but at times control measures are undertaken to preserve a species or to hold its population within bounds. Game management is the term applied to the production and harvesting of animal for sport. However, wildlife management is a broader term applying to all species of wild animal life, including birds, furbearers and fish. Wildlife depends on the vegetation for its existence. The type of plant formation and its stage in ecological succes- sion determine the species and number of animals which can occupy a habitat. Such species as mouse, elk, black- tailed deer, fox, lynx and beaver are most often found in the early deciduous tree and shrub phases of natural succession. In the sub-climax and climax stages, these animals occur mainly along the forest’s edge, where the mature climax forest joins parkland, alpine, grassland, or smaller openings, such as rivers, lakes, swamps, fields, and recently logged areas. -

Biodiversity Conservation for Sustainable Agriculture in Tropical Rainforest of Nigeria
New York Science Journal 2009;2(7), Emma-Okafor, et al Biodiversity Conservation for Sustainable Agriculture Biodiversity Conservation for Sustainable Agriculture in Tropical Rainforest of Nigeria. Emma-Okafor, Lilian Chinenye; Ibeawuchi, Izuchukwu Innocent, Obiefuna Julius Chiedozie Department of Crop Science and Technology Federal University of Technology , Owerri PMB 1526, Owerri, Imo State, Nigeria. Email: [email protected], [email protected], [email protected]. Abstract In this article we reviewed bio-conservation degradation in our ecosystem and its importance for sustainable agriculture in the tropical rainforest zone of Nigeria. Books, journals and newspapers were used. Our findings showed that the conservation of biodiversity degradation in our ecosystem is essentially important since mankind depends largely on livelihood derived from the natural resources such as land, forest, water and air. Also agricultural system functioning and sustainability depend greatly on it. Biodiversity degradation is facilitated due to various natural physical and biological factors such as population pressure, climate change, pollution, deforestation, soil Erosion, Habitat destruction, and introduction of exotic species, genetic erosion and poor law enforcement. Conservation of biodiversity could be achieved through various strategies such as creation of awareness on the need to preserve biological reserves or gene bank, market oriented approach, use of farmer’s indigenous knowledge and research based strategies. These measures will conserve and sustain Biodiversity and ensure enhanced food and health security for all. The integrated approach where scientists from all areas of biological science and agriculture put heads together to find a lasting solution to this cankerworm called biodiversity depreciation will make our ecosystem a better resource base for sustainable livelihood. -

Ethnobotanical Survey of Flora and Fauna Species in Kainji Lake National Park New-Bussa Local Government Area, Niger State, Nigeria
Annual Research & Review in Biology 36(8): 120-131, 2021; Article no.ARRB.63994 ISSN: 2347-565X, NLM ID: 101632869 Ethnobotanical Survey of Flora and Fauna Species in Kainji Lake National Park New-Bussa Local Government Area, Niger State, Nigeria M. R. Umar1* 1Department of Forestry and Wildlife Management, School of Agriculture and Agricultural Technology, Modibbo Adama University of Technology, Yola, Nigeria. Author’s contribution The sole author designed, analysed, interpreted and prepared the manuscript. Article Information DOI: 10.9734/ARRB/2021/v36i830417 Editor(s): (1) Dr. Manikant Tripathi , Dr. Ram Manohar Lohia Avadh University, India. Reviewers: (1) Muhammad Farooq Azhar , Bahauddin Zakariya University, Pakistan. (2) Pampi Ghosh, Vidyasagar University, India. Complete Peer review History: https://www.sdiarticle4.com/review-history/63994 Received 20 October 2020 Accepted 26 December 2020 Original Research Article Published 01 September 2021 ABSTRACT This study was carried out to identify the Ethnobotanical uses of flora and fauna species in Kainji Lake National Park New-Bussa Local Government Area, Niger State, Nigeria. The study objectives were to document knowledge and generate data on identification and uses of Plant species and animal species in Borgu and Zugurma sector of the park. Ethnobotanical surveys based on interviews from the park ranges, plant taxonomist and zoologist staff of the park and Botanical survey was carried out based on sample plots. A stratified random sampling was used to select sample sites within the two sector of the Park, based on flora and fauna sub-classification. A total Ten (10) plots of 100m x 100m strips were demarcated within the two sectors.