Antoine-Laurent Lavoisier
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metric System Units of Length
Math 0300 METRIC SYSTEM UNITS OF LENGTH Þ To convert units of length in the metric system of measurement The basic unit of length in the metric system is the meter. All units of length in the metric system are derived from the meter. The prefix “centi-“means one hundredth. 1 centimeter=1 one-hundredth of a meter kilo- = 1000 1 kilometer (km) = 1000 meters (m) hecto- = 100 1 hectometer (hm) = 100 m deca- = 10 1 decameter (dam) = 10 m 1 meter (m) = 1 m deci- = 0.1 1 decimeter (dm) = 0.1 m centi- = 0.01 1 centimeter (cm) = 0.01 m milli- = 0.001 1 millimeter (mm) = 0.001 m Conversion between units of length in the metric system involves moving the decimal point to the right or to the left. Listing the units in order from largest to smallest will indicate how many places to move the decimal point and in which direction. Example 1: To convert 4200 cm to meters, write the units in order from largest to smallest. km hm dam m dm cm mm Converting cm to m requires moving 4 2 . 0 0 2 positions to the left. Move the decimal point the same number of places and in the same direction (to the left). So 4200 cm = 42.00 m A metric measurement involving two units is customarily written in terms of one unit. Convert the smaller unit to the larger unit and then add. Example 2: To convert 8 km 32 m to kilometers First convert 32 m to kilometers. km hm dam m dm cm mm Converting m to km requires moving 0 . -

The Reality of Phlogiston in Great Britain
The Reality of Phlogiston in Great Britain John Stewart Abstract: Mi Gyung Kim (2008) has challenged the historiographical assump- tion that phlogiston was the paradigmatic concept in eighteenth century chemistry. Her analysis of the operational, theoretical, and philosophical iden- tities of phlogiston demonstrates how Stahlian phlogiston was appropriated into the burgeoning field of affinity theory. However, this new French con- ception of phlogiston was destabilized by the introduction of Boerhaave’s thermometrics. By extending this story through 1790, I will show that British pneumatic chemists integrated new understandings of heat with an affinity based operational definition of phlogiston and thereby stabilized the concept. What resulted was a new and very different phlogiston. Keywords: Eighteenth-century chemistry, Chemical Revolution, historical ontolo- gy, Richard Kirwan, phlogiston, fire . 1. Introduction Phlogiston has long been regarded as the paradigmatic concept in eighteenth- century chemistry. In his classic work, A Short History of Chemistry (1939), J. R. Partington attributed the development of phlogiston to the German met- allurgical chemists Johann Joachim Becher (1635-1682) and Georg Ernst Stahl (1660-1734). This phlogiston was analogous to Aristotle’s elemental fire in its roles in creating heat, light, and fire. Though conceptions of phlo- giston changed over the course of the century, it could generally be defined operationally as that which “escapes from burning bodies in a rapid whirling motion, and is contained in all combustible bodies and also in metals” (Kuhn 1962, p. 87). The roasting or calcination of metals was explained as the sepa- ration of phlogiston from the metallic calx. By 1720, French chemist Étienne- François Geoffroy (1672-1731) had appropriated phlogiston, identifying it with Homberg’s sulfurous principle (Kim 2008, pp. -

Gravity and Coulomb's
Gravity operates by the inverse square law (source Hyperphysics) A main objective in this lesson is that you understand the basic notion of “inverse square” relationships. There are a small number (perhaps less than 25) general paradigms of nature that if you make them part of your basic view of nature they will help you greatly in your understanding of how nature operates. Gravity is the weakest of the four fundamental forces, yet it is the dominant force in the universe for shaping the large-scale structure of galaxies, stars, etc. The gravitational force between two masses m1 and m2 is given by the relationship: This is often called the "universal law of gravitation" and G the universal gravitation constant. It is an example of an inverse square law force. The force is always attractive and acts along the line joining the centers of mass of the two masses. The forces on the two masses are equal in size but opposite in direction, obeying Newton's third law. You should notice that the universal gravitational constant is REALLY small so gravity is considered a very weak force. The gravity force has the same form as Coulomb's law for the forces between electric charges, i.e., it is an inverse square law force which depends upon the product of the two interacting sources. This led Einstein to start with the electromagnetic force and gravity as the first attempt to demonstrate the unification of the fundamental forces. It turns out that this was the wrong place to start, and that gravity will be the last of the forces to unify with the other three forces. -

Chemical Innovation in Plant Nutrition in a Historical Continuum from Ancient Greece and Rome Until Modern Times
DOI: 10.1515/cdem-2016-0002 CHEM DIDACT ECOL METROL. 2016;21(1-2):29-43 Jacek ANTONKIEWICZ 1* and Jan ŁAB ĘTOWICZ 2 CHEMICAL INNOVATION IN PLANT NUTRITION IN A HISTORICAL CONTINUUM FROM ANCIENT GREECE AND ROME UNTIL MODERN TIMES INNOWACJE CHEMICZNE W OD ŻYWIANIU RO ŚLIN OD STARO ŻYTNEJ GRECJI I RZYMU PO CZASY NAJNOWSZE Abstract: This monograph aims to present how arduously views on plant nutrition shaped over centuries and how the foundation of environmental knowledge concerning these issues was created. This publication also presents current problems and trends in studies concerning plant nutrition, showing their new dimension. This new dimension is determined, on one hand, by the need to feed the world population increasing in geometric progression, and on the other hand by growing environmental problems connected with intensification of agricultural production. Keywords: chemical innovations, plant nutrition Introduction Plant nutrition has been of great interest since time immemorial, at first among philosophers, and later among researchers. The history of environmental discoveries concerning the way plants feed is full of misconceptions and incorrect theories. Learning about the multi-generational effort to find an explanation for this process that is fundamental for agriculture shows us the tenacity and ingenuity of many outstanding personalities and scientists of that time. It also allows for general reflection which shows that present-day knowledge (which often seems obvious and simple) is the fruit of a great collective effort of science. Antiquity Already in ancient Greece people were interested in life processes of plants, the way they feed, and in the conditions that facilitate or inhibit their growth. -

The International Bureau of Weights and Measures 1875-1975
The International Bureau of Weights and Measures 1875-1975 U.S. DEPARTMENT OF COMMERCE National Bureau of Standards ""EAU of NBS SPECIAL PUBLICATION 420 Aerial view of the Pavilion de Breteuil and the immediate environs. To the east, the Seine and the Pont de Sevres; to the northwest, the Pare de Saint-Cloud: between the Pavilion de Breteuil (circled) and the bridge: the Manufacture Nationale de Porcelaine de Sevres. The new laboratories (1964) are situated north of the circle and are scarcely visible; they were built in a way to preserve the countryside. (Document Institute (leographique National, Paris). Medal commeiiKiraUn-i the centennial (if the Convention cif tlie Metre and the International Bureau of Weights and Measures. (Desifined by R. Corbin. Monnaie de Paris) The International Bureau of Weights and Measures 1875-1975 Edited by Chester H. Page National Bureau of Standards, U.S.A. and Pan I Vigoiireiix National Physieal Laboratory, U.K. Translation of tlie BIPM Centennial Volume Piibli>lieH on the ocrasioii <>( the lOOth Aniiiver^ai y ol tlie Treaty of tlie Metre May 20, 1975 U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS, Richard W. Roberts, Direcior Issued May 1 975 National Bureau of Standards Special Publication 420 Nat. Bur. Stand. (U.S.), Spec. Publ. 420. 256 pages (May 1975) CODEN: XNBSAV U.S. GOVERNMENT PRINTING OFFICE WASHINGTON: 1975 For sale by the Superintendent of Documents U.S. Government Printing Office, Washington, D.C. 20402 Paper cover Price $3.00 Stock Number 003-003-01408 Catalog Number C13.10:420 FOREWORD The metric system was made legal by Congress in 1866, the United States of America signed the Treaty of the Metre in 1875, and we have been active in international coordination of measurements since that time. -
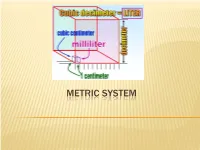
Metric System.Pdf
METRIC SYSTEM THE METRIC SYSTEM The metric system is much easier. All metric units are related by factors of 10. Nearly the entire world (95%), except the United States, now uses the metric system. Metric is used exclusively in science. Because the metric system uses units related by factors of ten and the types of units (distance, area, volume, mass) are simply-related, performing calculations with the metric system is much easier. METRIC CHART Prefix Symbol Factor Number Factor Word Kilo K 1,000 Thousand Hecto H 100 Hundred Deca Dk 10 Ten Base Unit Meter, gram, liter 1 One Deci D 0.1 Tenth Centi C 0.01 Hundredth Milli M 0.001 Thousandth The metric system has three units or bases. Meter – the basic unit used to measure length Gram – the basic unit used to measure weight Liter – the basic unit used to measure liquid capacity (think 2 Liter cokes!) The United States, Liberia and Burma (countries in black) have stuck with using the Imperial System of measurement. You can think of “the metric system” as a nickname for the International System of Units, or SI. HOW TO REMEMBER THE PREFIXES Kids Kilo Have Hecto Dropped Deca Over base unit (gram, liter, meter) Dead Deci Converting Centi Metrics Milli Large Units – Kilo (1000), Hecto (100), Deca (10) Small Units – Deci (0.1), Centi (0.01), Milli (0.001) Because you are dealing with multiples of ten, you do not have to calculate anything. All you have to do is move the decimal point, but you need to understand what you are doing when you move the decimal point. -

Rtusaeatz Nt,Rnasiivene
THE EVENING STAR. 38488 RQQe PUBLISRIED I AILY, Except Sunday, AT THE STAR BUILDINGS, any mznslo to sen e ear.te war to Avene, Corner 11th Street, 0011T the2ing f 1a0 tothi6 clany, it is Pensaylrania by partinenty as And why aboud as me '1he Evening Star Newspaper Oompany. King at sia1 reCeve the same alten as did 4:EQRUE W. ADAMS, Pres'r. one of his subjeCm alG the representative of a Tn EvrtNan Sra i. P.red to niscribers in the royal line, who, being aocredite to the paent r ty l. ,arr rP, on ti.eir own ateount, at 10 eents amInstmration, was, at the requet of Mr. 1 er w.ak or 44 eente per wonth. Colies at the Sicmes, our consul at Bangkok, sent from Siam eT:nt; r. - cnte each. by wail-sjotaKe prel aid- on one of our naval veeea, was received by toa eente a Llouth ; ene ye,r, $6; six months, I3. the iEterPd at the Poet Office t Washington, 0., President and Mrs. Hayes at the White -ase no. ca- mail ntatter 1 Housewith effusion, entertained there by them 'Ia V.1KT ST-r-p>b ished on F.atay-*2 a and mated asa member of thefamily for a con- errst trive irni;+i. Six months, 4; 10 copies siderable lenah of time? Every attention was for j1.: 20 col, . f.r $.. ARCH lavished on this myal guest; and should not the WAll nail ";bsCriltloine meit be paid in ad- CEN handsome young King receive as much? Yet It nee; > pa r ent l.ug than o paid for. -

Section 1 – Oxygen: the Gas That Changed Everything
MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS 1. Oxygen: The Gas that Changed Everything CHAPTER 1: What is the World Made Of? Alignment with the NRC’s National Science Education Standards B: Physical Science Structure and Properties of Matter: An element is composed of a single type of atom. G: History and Nature of Science Nature of Scientific Knowledge Because all scientific ideas depend on experimental and observational confirmation, all scientific knowledge is, in principle, subject to change as new evidence becomes available. … In situations where information is still fragmentary, it is normal for scientific ideas to be incomplete, but this is also where the opportunity for making advances may be greatest. Alignment with the Next Generation Science Standards Science and Engineering Practices 1. Asking Questions and Defining Problems Ask questions that arise from examining models or a theory, to clarify and/or seek additional information and relationships. Re-enactment: In a dank alchemist's laboratory, a white-bearded man works amidst a clutter of Notes from the Field: vessels, bellows and furnaces. I used this section of the program to introduce my students to the concept of atoms. It’s a NARR: One night in 1669, a German alchemist named Hennig Brandt was searching, as he did more concrete way to get into the atomic every night, for a way to make gold. theory. Brandt lifts a flask of yellow liquid and inspects it. Notes from the Field: Humor is a great way to engage my students. NARR: For some time, Brandt had focused his research on urine. He was certain the Even though they might find a scientist "golden stream" held the key. -

Recruitment of LC3 to Damaged Golgi Apparatus
Cell Death & Differentiation (2019) 26:1467–1484 https://doi.org/10.1038/s41418-018-0221-5 ARTICLE Recruitment of LC3 to damaged Golgi apparatus 1,2,3 4 2,3 2,3 5,6 Lígia C. Gomes-da-Silva ● Ana Joaquina Jimenez ● Allan Sauvat ● Wei Xie ● Sylvie Souquere ● 4 7 8,9 8,9 1 2,3 Séverine Divoux ● Marko Storch ● Baldur Sveinbjørnsson ● Øystein Rekdal ● Luis G. Arnaut ● Oliver Kepp ● 2,3,10,11,12,13 4 Guido Kroemer ● Franck Perez Received: 11 May 2018 / Accepted: 8 October 2018 / Published online: 22 October 2018 © ADMC Associazione Differenziamento e Morte Cellulare 2018 Abstract LC3 is a protein that can associate with autophagosomes, autolysosomes, and phagosomes. Here, we show that LC3 can also redistribute toward the damaged Golgi apparatus where it clusters with SQSTM1/p62 and lysosomes. This organelle-specific relocation, which did not involve the generation of double-membraned autophagosomes, could be observed after Golgi damage was induced by various strategies, namely (i) laser-induced localized cellular damage, (ii) local expression of peroxidase and exposure to peroxide and diaminobenzidine, (iii) treatment with the Golgi-tropic photosensitizer redaporfin and light, (iv) or exposure to the Golgi-tropic anticancer peptidomimetic LTX-401. Mechanistic exploration led to the conclusion that both reactive oxygen species-dependent and -independent Golgi damage induces a similar phenotype that 1234567890();,: 1234567890();,: depended on ATG5 yet did not depend on phosphatidylinositol-3-kinase catalytic subunit type 3 and Beclin-1. Interestingly, knockout of ATG5 sensitized cells to Golgi damage-induced cell death, suggesting that the pathway culminating in the relocation of LC3 to the damaged Golgi may have a cytoprotective function. -

Ley De Conservación De La Masa
CULTURA CIENTÍFICA para la Enseñanza Secundaria LEY DE CONSERVACIÓN DE LA MASA: DE LA ALQUIMIA A LA QUÍMICA MODERNA Antoine Laurent Lavoisier Este trabajo ha sido realizado en el marco de un proyecto de investigación docente conce- dido y financiado por el Vicerrectorado de Es- tudiantes y Acción Social y el Vicerrectorado de Investigación de la Universidad Católica de Valencia San Vicente Mártir. CULTURA CIENTÍFICA para la Enseñanza Secundaria Con este proyecto se pretende que los alum- nos de Enseñanza Secundaria Obligatoria (E.S.O.) adquieran una cultura científica y co- Edita: nozcan que la ciencia, la sociedad y la tecno- UNIVERSIDAD CATÓLICA DE VALENCIA logía no se pueden concebir aisladamente. Vicerrectorado de Estudiantes y Acción Social Alumnos y profesores hemos trabajado desde Vicerrectorado de Investigación una perspectiva multidisciplinar a través de Diseño y Maquetación: diferentes asignaturas y Grados Universitarios. Medianil Comunicación / medianil.com Autores: Stephany Cuellar Mosquera Sergio Gómez Molina Mariana Herrán Gonzalez Rocío Navarro Salazar Javier Pérez Murillo María Rojas Chacón José Urpin Rangel Asignatura: Fundamentos básicos de Química Profesor/a: Dra. Ángela Moreno Gálvez Grado: Nutrición Humana y Dietética Coordinadora: Dra. Gloria Castellano Estornell ÉRASE UNA VEZ 3 Descubrimiento de la Ley de conservación de la masa Antes de Lavoisier la Química como de los productos de una reacción ciencia apenas existía. Los cono- química debe ser igual al peso de cimientos que había eran vagos los reactantes, estableciendo que y estaban incluso en ocasiones “la materia ni se crea ni se destru- mezclados con conceptos cercanos ye en cualquier reacción química”, a lo mágico o lo esotérico, según y transformando así la química en la tradición alquímica provenien- ciencia con mayúsculas. -

Report to AID on a Philippines Survey on Standardization and Measurement Services
TECH NATL INST OF STAND & NIST PUBLICATIONS A111D? OSfilb^ IMBSIR 76-1083 Report to AID on a Philippines Survey on Standardization and Measurement Services Edited by: H. Steffen Peiser Robert S. Marvin Office of International Relations National Bureau of Standards Washington, D. C. 20234 Conducted May 4 17, 1975 Issued June 1 976 The Survey was conducted as a part of the program under the US/NBS/Agency for International Development PASA TA(CE) 5-71 \ epared for gency for International Development * 7L'/b83 epartment of State jCj^ Washington, D. C. 20523 NBSIR 76-1083 REPORT TO AID ON A PHILIPPINES SURVEY ON STANDARDIZATION AND MEASUREMENT SERVICES Edited by: H. Steffen Peiser Robert S. Marvin Office of International Relations National Bureau of Standards Washington, D. C. 20234 Conducted May 4 - 17, 1975 Issued June 1 976 The Survey was conducted as a part of the program under the US/NBS/Agency for International Development PASA TA(CE) 5-71 Prepared for Agency for International Development Department of State Washington, D. C. 20523 U.S. DEPARTMENT OF COMMERCE, Elliot L. Richardson, Secretary Dr. Betsy Ancfcer-Johrtsor At**st*nt Secretly for Science end Technology NATIONAL BUREAU OF STANDARDS. Ernest Ambler. Acting Director i TABLE OF CONTENTS Paee PARTICIPANTS 1 I INTRODUCTION 4 II RECOMMENDATIONS - SUMMARIZED 6 III THE JOINT PROGRAM OF THE SURVEY TEAM 12 IV REPORT OF GROUP A, TECHNICAL STANDARDS COMMITTEE MANAGEMENT, Group Leader; Dr. Kenneth S. Stephens, School of Industrial & Systems Engineering and Industrial Development Division, Engr. Exp. Station, Georgia Institute of Technology, Atlanta, Georgia 29 V REPORT OF GROUP B, METRICATION Group Leader; Mr. -

LAVOISIER-The Crucial Year the Background and Origin of His First
LAVOISIER-THE CRUCIAL YEAR: The Background and Origin of His First Experiments on Combustion in z772 Antoine Laurent Lavoisier, 17 43-1794, a portrait by David (Photo Roger-Viollet) LA VOISIER -The Crucial Year The Background and Origin of His First Experiments on Combustion in 1772 /J_y llenr_y (Juerlac CORNELL UNIVERSITY CORNELL UNIVERSITY PRESS Ithaca, New York Open access edition funded by the National Endowment for the Humanities/Andrew W. Mellon Foundation Humanities Open Book Program. This work has been brought to publication with the assistance of a grant from the Ford Foundation. Copyright © 1961 by Cornell University First paperback printing 2019 The text of this book is li censed under a Creative Commons Attribution- NonCommerciai-NoDerivatives 4.0 International License: https://creativecommons.org/licenses/by-nc-nd/4.0/. To use this book, or parts of this book, in any way not covered by the li cense, please contact Cornell University Press, Sage House, 512 East State Street, Ithaca, New York 14850. Visit our website at cornellpress.cornell.edu. Printed in the United States of America ISBN 978- 1-501 7-4663-5 (pbk.: alk. paper) ISBN 978-1-5017-4664-2 (pdf) ISBN 978-1-5017-4665-9 ( epub/mobi) Librarians: A CIP catalog record for this book is available from the Library of Congress TO Andrew Norman Meldrum (1876-1934) AND Helene Metzger (188g-1944) Acknowledgments MUCH of the research and much of the writing of a first draft of this book was completed while I was a mem ber of the Institute for Advanced Study, Princeton, in 1953-1955.