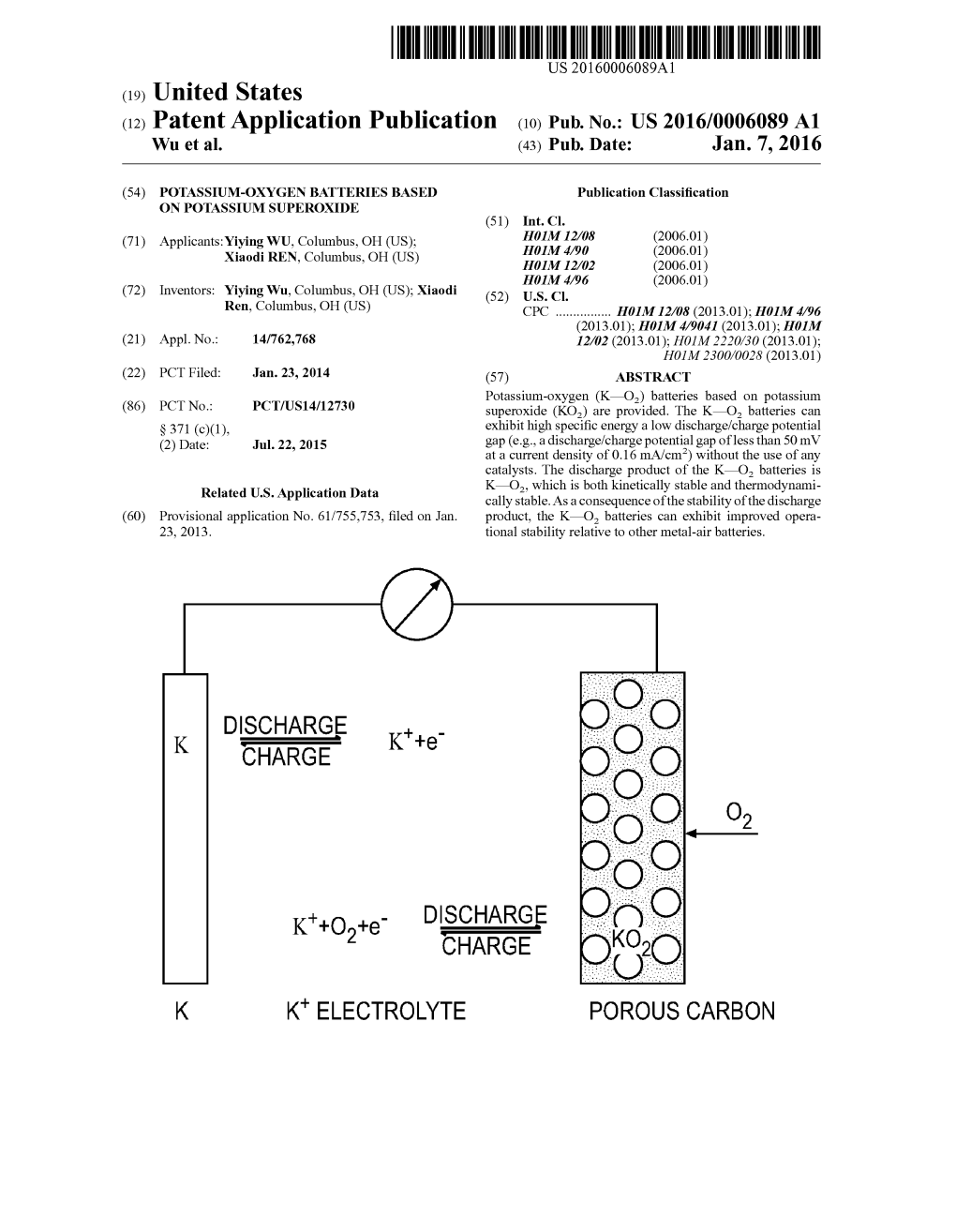
K"+O+E DISCHARGE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nomination Background: Methylal (CASRN: 109-87-5)
SUMMARY OF DATA FOR CHEMICAL SELECTION METHYLAL CAS NO. 109-87-5 BASIS OF NOMINATION TO THE CSWG The nomination of methylal to the CSWG is based on high production volume and exposure potential. Dr. Elizabeth Weisburger, a member of the American Conference of Governmental Industrial Hygienists (ACGIH) TLV Committee as well as the Chemical Selection Working Group (CSWG), provided a list of 281 chemical substances with ACGIH recommended TLVs for which there were no long term studies cited in the supporting data and no designations with respect to carcinogenicity. She presented the list to the Chemical Selection Planning Group (CSPG) for evaluation as chemicals which may warrant chronic testing: it was affirmed at the CSPG meeting held on August 9, 1994, that the 281 "TLV Chemicals" be reviewed as a Class Study. As a result of the class study review, methylal is presented as a candidate for testing by the National Toxicology Program because of: • potential for occupational exposures based on high production volume (1.2-64 million lbs) and estimate of worker exposure • evidence of occupational exposures based on TLV and other literature documentation • potential for general population exposures based on use as a solvent in consumer products and occurrence in environmental media • suspicion of carcinogenicity based on potential for metabolic release of formaldehyde and positive mutagenicity data • lack of chronic toxicity data. SELECTION STATUS ACTION BY CSWG : 9/25/96 Studies requested : - Carcinogenicity Priority : Moderate to High Rationale/Remarks : - Potential for human exposure - Inhalation route recommended for testing - Consider transgenic mouse model (p53 or TGAC) INPUT FROM GOVERNMENT AGENCIES/INDUSTRY Dr. -

Cyclopentyl Methyl Ether (CPME)
Korean J. Chem. Eng., 34(2), 463-469 (2017) pISSN: 0256-1115 DOI: 10.1007/s11814-016-0265-5 eISSN: 1975-7220 INVITED REVIEW PAPER Measurement and correlation of the isothermal VLE data for the binary mixtures of cyclopentene (CPEN)+cyclopentyl methyl ether (CPME) Wan Ju Jeong and Jong Sung Lim† Department of Chemical and Biomolecular Engineering, Sogang University, C.P.O. Box 1142, Seoul 04107, Korea (Received 2 August 2016 • accepted 20 September 2016) Abstract−The isothermal vapor-liquid equilibrium data for the binary systems of cyclopentene (1)+cyclopentyl methyl ether (2) were measured at 313.15, 323.15, 333.15, 343.15 and 353.15 K using a dynamic-type equilibrium apparatus and online gas chromatography analysis. For all the measured VLE data consistency tests were performed for the verifi- cation of data using Barker’s method and the ASPEN PLUS Area Test method. All the resulting average absolute val- δ γ γ ues of residuals [ ln ( 1/ 2)] for Barker’s method and D values for the ASPEN PLUS area test method were com- paratively small. So, the VLE data reported in this study are considered to be acceptable. This binary system shows neg- ative deviation from Raoult’s law and does not exhibit azeotropic behavior at whole temperature ranges studied here. The measured data were correlated with the P-R EoS using the Wong-Sandler mixing rule. The overall average relative deviation of pressure (ARD-P (%)) between experimental and calculated values was 0.078% and that of vapor phase compositions (ARD-y (%)) was 0.452%. Keywords: Vapor Liquid Equilibria (VLE), Cyclopentyl Methyl Ether (CPME), Cyclopentene (CPEN), Peng-Robinson Equation of State (PR-EoS), Wong-Sandler Mixing Rule (WS-MR) INTRODUCTION however, have some disadvantages because they use dimethyl sul- fate and methyl iodide as a reactant, respectively, which are muta- Ethers are widely used in organic chemistry and biochemistry. -

Guidance Document Peroxide-Forming Chemicals
Guidance Document Peroxide-Forming Chemicals Some chemicals can form peroxides under normal storage conditions. Some of the peroxide chemicals are unstable, especially when dried or concentrated, and can explode violently when subjected to heat, light or mechanical shock. In addition, some of the inadvertently formed peroxides can initiate other unexpected violent reactions (e.g. polymerizations) with other chemicals. When possible and practical for your work, purchase chemicals that have inhibitors added by the manufacturer. Label peroxide-forming chemicals with date received and date opened. Store peroxide-formers in airtight opaque containers with screw caps. Consider oxygen exclusion methods such as purging with inert gas or sealing containers with parafilm. Inspect containers for signs of peroxide formation. Do not open a container which has crystals or a visible cloudiness. Call EHS to come remove it. The friction caused by opening a lid can cause an explosion. Liquids can be tested for presence of peroxide. This is especially important prior to distilation. Most explosions of peroxide forming chemicals occur when a material is distilled to dryness. Peroxide test kits are available from chemical vendors. Contact EHS for additional guidance. Classification Table for Peroxide-Forming Chemicals Class I:: Unsaturated materials, especially those of low molecular weight, may polymerize violently and hazardously due to peroxide initiation. These chemicals can spontaneously decompose, becoming explosive after exposure to air with concentration. Discard unopened containers within 3 months. Opened containers should be tested for peroxides every 2 months. Acrylic acid Tetrafluoroethylene Acrylonitrile Vinyl acetate 1,3-Butadiene Vinyl acetylene Chlorobutadiene (chloroprene) Vinyl chloride Chlorotrifluoroethylene Vinyl pyridine Methyl methacrylate Vinylidiene chloride Styrene Class II: The following chemicals are a peroxide hazard upon concentration (distillation/evaporation). -

NMR Chemical Shifts of Common Laboratory Solvents As Trace Impurities
7512 J. Org. Chem. 1997, 62, 7512-7515 NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities Hugo E. Gottlieb,* Vadim Kotlyar, and Abraham Nudelman* Department of Chemistry, Bar-Ilan University, Ramat-Gan 52900, Israel Received June 27, 1997 In the course of the routine use of NMR as an aid for organic chemistry, a day-to-day problem is the identifica- tion of signals deriving from common contaminants (water, solvents, stabilizers, oils) in less-than-analyti- cally-pure samples. This data may be available in the literature, but the time involved in searching for it may be considerable. Another issue is the concentration dependence of chemical shifts (especially 1H); results obtained two or three decades ago usually refer to much Figure 1. Chemical shift of HDO as a function of tempera- more concentrated samples, and run at lower magnetic ture. fields, than today’s practice. 1 13 We therefore decided to collect H and C chemical dependent (vide infra). Also, any potential hydrogen- shifts of what are, in our experience, the most popular bond acceptor will tend to shift the water signal down- “extra peaks” in a variety of commonly used NMR field; this is particularly true for nonpolar solvents. In solvents, in the hope that this will be of assistance to contrast, in e.g. DMSO the water is already strongly the practicing chemist. hydrogen-bonded to the solvent, and solutes have only a negligible effect on its chemical shift. This is also true Experimental Section for D2O; the chemical shift of the residual HDO is very NMR spectra were taken in a Bruker DPX-300 instrument temperature-dependent (vide infra) but, maybe counter- (300.1 and 75.5 MHz for 1H and 13C, respectively). -

Sure/Seal™ System for Anhydrous Solvents
QUALITY AND SAFETY. SEALED. Sure/Seal™ system for anhydrous solvents The life science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the U.S. and Canada. Choose from the largest range of high-quality anhydrous solvents Innovative plug style with exceptionally low water levels. • Maximum surface area contact (liner to bottle) to exclude Rest assured that each product moisture and oxygen is perfectly protected with our innovative, moisture-inhibiting • More than 50% thicker than competing brands to ensure low Sure/Seal™ system. We use three water content for entire shelf life different types of materials to ensure complete compatibility with Outstanding elastomer and crimp cap design contents, and easier handling for you. Sure/Seal™ bottles come in • Air-tight system to protect product quality several sizes, ranging from 100 mL • Excellent resealing properties to 2 L. • Secondary resin layer ensures resistance to chemicals • Outperforms competitors’ seals in moisture prevention • Three unique plug-style liners to suit a wide range of solvents and solutions Highest quality anhydrous solvents • Always maintains exceptionally low water content • More than 90 products in different categories, including common air and/or moisture-sensitive, volatiles, and strong odors • Various size offerings, from 100 mL to 2 L (Larger size of one- way container also available in North America) Three unique plug-style liners created for optimal compatibility with various solvents White liner for Hexane, Toluene, Dichloromethane Gray -

D0md00174k1.Pdf
Electronic Supplementary Material (ESI) for RSC Medicinal Chemistry. This journal is © The Royal Society of Chemistry 2020 Supporting Information Rational Design, Synthesis and Testing of Novel Tricyclic Topoisomerase Inhibitors for the Treatment of Bacterial Infections Part 1 R. Kirka, A. Ratcliffe, G. Noonan, M. Uosis-Martin, D. Lyth, O. Bardell-Cox, J. Massam, P. Schofield, S. Hindley, D. Jones, J. Maclean, A. Smith, V. Savage, S. Mohmed, C. Charrier, A-M. Salisbury, E. Moyo, R. Metzger, N. Chalam- Judge, J. Cheung, N. R. Stokes, S. Best, M. Craighead, R. Armer, A. Huxley Index: General Experimental Procedures S2- S3 Synthetic Procedures S4- S62 Antibacterial susceptibility testing protocols S63 References S63 1 Synthetic Procedures General Information All reactions were carried out using commercial materials and reagents without further purification unless otherwise noted. All reactions were monitored by thin layer chromatography (TLC). Visualization of the spots on TLC plates was achieved by UV light and by staining the TLC plates in potassium permanganate and charring with a heat gun, unless otherwise stated. NMR spectral data was recorded on a LC Bruker AV400 using a 5 mm QNP probe or Bruker AVIII 400 Nanobay using a 5 mm BBFQ with z-gradients. Chemical shifts are expressed in parts per million values (ppm) and are designated as s (singlet); br s (broad singlet); d (doublet); t (triplet); q (quartet); quint (quintet) or m (multiplet). Where appropriate, COSY and NOE experiments were carried out to aid assignment. Chromatography was performed on a an ISCO using silica (normal phase or C18 (reverse phase; or by flash-column chromatography using silica gel (Fluorochem silica gel 60A 40-63 µm). -

Influence of Nonaqueous Solvents on the Electrochemistry of Oxygen In
9178 J. Phys. Chem. C 2010, 114, 9178–9186 Influence of Nonaqueous Solvents on the Electrochemistry of Oxygen in the Rechargeable Lithium-Air Battery Cormac O. Laoire, Sanjeev Mukerjee, and K. M. Abraham* Department of Chemistry and Chemical Biology, Northeastern UniVersity, 360 Huntington AVenue, Boston, Massachusetts 02115 Edward J. Plichta and Mary A. Hendrickson U.S. Army CERDEC, Army Power DiVision, Ft. Monmouth, New Jersey 07703 ReceiVed: March 5, 2010; ReVised Manuscript ReceiVed: March 23, 2010 A fundamental study of the influence of solvents on the oxygen reduction reaction (ORR) in nonaqueous electrolytes has been carried out for elucidating the mechanism of the oxygen electrode processes in the rechargeable Li-air battery. Using either tetrabutylammonium hexafluorophosphate (TBAPF6) or lithium hexafluorophosphate (LiPF6) electrolyte solutions in four different solvents, namely, dimethyl sulfoxide (DMSO), acetonitrile (MeCN), dimethoxyethane (DME), and tetraethylene glycol dimethyl ether (TEGDME), possessing a range of donor numbers (DN), we have determined that the solvent and the supporting electrolyte cations in the solution act in concert to + influence the nature of reduction products and their rechargeability. In solutions containing TBA ,O2 reduction is - + a highly reversible one-electron process involving the O2/O2 couple. On the other hand, in Li -containing electrolytes - 2- 2- relevant to the Li-air battery, O2 reduction proceeds in a stepwise fashion to form O2 ,O2 , and O as products. These reactions in the presence of Li+ are irreversible or quasi-reversible electrochemical processes, and the solvents have significant influence on the kinetics, and reversibility or lack thereof, of the different reduction products. The - + stabilization of the one-electron reduction product, superoxide (O2 )inTBA solutions in all of the solvents examined + - can be explained using Pearson’s hard soft acid base (HSAB) theory involving the formation of the TBA ---O2 + complex. -

3704 Green Chemistry.Indd
Physical Properties of Solvents Properties CPME 2-MeTHF THF Ether DCM 1,4-Dioxane MTBE Stabilizer Required No Yes Yes Yes Yes No No Green Chemistry Density (20 °C) [g/cm3] 0.86 0.86 0.89 0.71 1.32 1.03 0.74 Dielectric constant (25 °C) 4.76 6.97 7.58 4.197 8.93 2.227 — Supporting the Advancement of Chemistry Boiling point [°C] 106 80 65 34.6 39.8 101 55 Heat of Vaporization 69.2 87.1 98.1 86.1 80.5 98.6 81.7 through Sound Environmental, Social, and (bp)[Kcal/kg] Solubility of Solvent in Water Fiscal Responsibilities 1.1 4.1 Infi nite 6.5 1.3 Infi nite 4.8 (23 °C) [g/100g] Solubility of Water in Solvent 0.3 14.4 Infi nite 1.2 0.2 Infi nite 1.5 (23 °C) [g/100g] Azeotropic temperature with 83 89 64 34 39 88 52 Water [°C] Flash point [°C] –1 –11.1 –14.2 –45 — 12 –28 Explosion range [vol%] 1.1% / 9.9% — 1.84% / 11.8% 1.85% / 48% 14% / 22% 2% / 22% 1.6% / 15.1% Lower / Upper limit Peroxide Formation of Ether Solvent 250 200 150 100 Peroxide wt ppm 50 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Days (x) CPME THF Stabilized THF Rate of Peroxide Formation by days (x) Y = 0.2033x Y = 0.8218x Y = 15.277e0.0962x Conditions - 20 mL of each sample in a brown bottle (capacity of 65 mL) - Stored at room temperature, in a dark place and in the presence of air. -

Abbreviations and Acronyms
The Journal of Organic Chemistry Standard Abbreviations and Acronyms observed optical rotation in degrees cm–1 wavenumber(s) [] specific rotation [expressed without cod 1,5-cyclooctadiene units; the units, (degmL)/(gdm), compd compound are understood] concd concentrated Å angstrom(s) concn concentration Ac acetyl COSY correlation spectroscopy acac acetylacetonate cot 1,3,5,7-cyclooctatetraene ADP adenosine 5-diphosphate Cp cyclopentadienyl AIBN 2,2-azobisisobutyronitrile m-CPBA meta-chloroperoxybenzoic acid AM1 Austin model 1 CV cyclic voltammetry AMP adenosine 5-monophosphate chemical shift in parts per million Anal. combustion elemental analysis downfield from tetramethylsilane anhyd anhydrous d day(s); doublet (spectral); deci AO atomic orbital d density aq aqueous DABCO 1,4-diazabicyclo[2.2.2]octane Ar aryl dansyl 5-(dimethylamino)- atm atmosphere(s) 1-naphthalenesulfonyl DBN 1,5-diazabicyclo[4.3.0]non-5-ene ATP adenosine 5-triphosphate DBU 1,8-diazabicyclo[5.4.0]undec-7-ene ATPase adenosinetriphosphatase av average DCC N,N-dicyclohexylcarbodiimide 9-BBN 9-borabicyclo[3.3.1]nonyl DCE 1,2-dichloroethane 9-BBN–H 9-borabicyclo[3.3.1]nonane DDQ 2,3-dichloro-5,6-dicyano- Bn, Bzl benzyl 1,4-benzoquinone DEAD diethyl azodicarboxylate bpy 2,2-bipyridyl BOC, Boc tert-butoxycarbonyl DEPT distortionless enhancement by bp boiling point, base pair polarization transfer br broad (spectral) DFT density functional theory Bu, n-Bu normal (primary) butyl DIBALH diisobutylaluminum hydride s-Bu sec-butyl DMA dimethylacetamide DMAP 4-(N,N-dimethylamino)pyridine -

Q3C (R8) Step 5
20 May 2021 EMA/CHMP/ICH/82260/2006 Committee for Medicinal Products for Human Use ICH guideline Q3C (R8) on impurities: guideline for residual solvents Step 5 Transmission to CHMP 30 April 2020 Adoption by CHMP 30 April 2020 Release for public consultation 4 May 2020 Deadline for comments 30 July 2020 Final adoption by CHMP 20 May 2021 Date for coming into effect 20 November 2021 Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2021. Reproduction is authorised provided the source is acknowledged. Document History Code History Date Parent Guideline: Impurities: Guideline for Residual Solvents Q3C Approval by the Steering Committee under Step 2 and 6 November 1996 release for public consultation. Q3C Approval by the Steering Committee under Step 4 and 17 July 1997 recommendation for adoption to the three ICH regulatory bodies. Revision of the PDE information for THF contained in the Parent Guideline Q3C(R1) Permissible Daily Exposure (PDE) for Tetrahydrofuran 20 July 2000 (THF): revision of PDE based on new toxicological Note: Prior data. to adding the revision to Approval by the Steering Committee of the new PDE the parent for THF under Step 2 and release for public Guideline in consultation. November 2005, the code was Q3C(M) for THF. Q3C(R1) Approval by the Steering Committee under Step 4 and 12 September recommendation for adoption to the three ICH 2002 Note: Prior regulatory bodies. -

Peptides and Glycine Derivatives Via Direct C–H Bond Functionalization
Site-specific C-functionalization of free-(NH) peptides and glycine derivatives via direct C–H bond functionalization Liang Zhao, Oliver Basle´ , and Chao-Jun Li1 Department of Chemistry, McGill University, 801 Sherbrooke Street West, Montreal, Quebec H3A 2K6, Canada. Edited by Jack Halpern, University of Chicago, Chicago, IL, and approved January 14, 2009 (received for review September 11, 2008) A copper-catalyzed ␣-functionalization of glycine derivatives and short peptides with nucleophiles is described. The present method provides ways to introduce functionalities such as aryl, vinyl, alkynyl, or indolyl specifically to the terminal glycine moieties of small pep- tides, which are normally difficult in peptide modifications. Further- more, on functionalization, the configurations of other stereocenters in the peptides could be maintained. Because the functionalized Fig. 1. C-functionalization of N terminus of peptides. peptides could easily be deprotected and carried onto the next coupling process, our approach provides a useful tool for the peptide- based biological research. globally in the year 2000 (29). Although the Strecker synthesis (30–32), the Ugi reaction (33–36) and the Petasis reaction (37–39) amino acid ͉ C–C bond formation ͉ peptide modification are important tools to construct arylglycine derivatives; direct arylation of glycine derivatives or glycine moieties in peptides would ecent advances in proteomics demands innovative methods to be more powerful in cases where the glycine moiety is already Rrapidly generate and modify peptides and amino acids. Direct present. Herein, we wish to report the detailed study of a general and site-specific modification of amino acids and peptides takes method for site-specific C arylation, vinylation, alkynylation, and CHEMISTRY advantage of the existing structure and provides a convenient way indolylation of ␣-C–H bonds of glycines and short peptides at the to generate large arrays of diverse amino acids and peptides for N terminus (Fig. -

Evaluating Green Solvents and Techniques in Extraction Methods
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange Electronic Theses and Dissertations 2019 Evaluating Green Solvents and Techniques in Extraction Methods Shanmugapriya Dharmarajan South Dakota State University Follow this and additional works at: https://openprairie.sdstate.edu/etd Part of the Analytical Chemistry Commons Recommended Citation Dharmarajan, Shanmugapriya, "Evaluating Green Solvents and Techniques in Extraction Methods" (2019). Electronic Theses and Dissertations. 3658. https://openprairie.sdstate.edu/etd/3658 This Dissertation - Open Access is brought to you for free and open access by Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. For more information, please contact [email protected]. EVALUATING GREEN SOLVENTS AND TECHNIQUES IN EXTRACTION METHODS BY SHANMUGAPRIYA DHARMARAJAN A dissertation submitted in partial fulfillment of the requirements for the Doctor of Philosophy Major in Chemistry South Dakota State University 2019 DocuSign Envelope ID: 40A68B4B-711B-4A10-BF3F-6DC84EC68C91 ii DISSERTATION ACCEPTANCE PAGE Shanmugapriya Dharmarajan This dissertation is approved as a creditable and independent investigation by a candidate for the Doctor of Philosophy degree and is acceptable for meeting the dissertation requirements for this degree. Acceptance of this does not imply that the conclusions reached by the candidate are necessarily the conclusions of the major department. Douglas Raynie Advisor Date Department Head Date Dean, Graduate School Date iii ACKNOWLEDGEMENTS I express my deepest gratitude to my advisor, Dr. Douglas Raynie, for his relentless support, constant encouragement and guidance throughout this journey.