Cyclopentyl Methyl Ether (CPME)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nomination Background: Methylal (CASRN: 109-87-5)
SUMMARY OF DATA FOR CHEMICAL SELECTION METHYLAL CAS NO. 109-87-5 BASIS OF NOMINATION TO THE CSWG The nomination of methylal to the CSWG is based on high production volume and exposure potential. Dr. Elizabeth Weisburger, a member of the American Conference of Governmental Industrial Hygienists (ACGIH) TLV Committee as well as the Chemical Selection Working Group (CSWG), provided a list of 281 chemical substances with ACGIH recommended TLVs for which there were no long term studies cited in the supporting data and no designations with respect to carcinogenicity. She presented the list to the Chemical Selection Planning Group (CSPG) for evaluation as chemicals which may warrant chronic testing: it was affirmed at the CSPG meeting held on August 9, 1994, that the 281 "TLV Chemicals" be reviewed as a Class Study. As a result of the class study review, methylal is presented as a candidate for testing by the National Toxicology Program because of: • potential for occupational exposures based on high production volume (1.2-64 million lbs) and estimate of worker exposure • evidence of occupational exposures based on TLV and other literature documentation • potential for general population exposures based on use as a solvent in consumer products and occurrence in environmental media • suspicion of carcinogenicity based on potential for metabolic release of formaldehyde and positive mutagenicity data • lack of chronic toxicity data. SELECTION STATUS ACTION BY CSWG : 9/25/96 Studies requested : - Carcinogenicity Priority : Moderate to High Rationale/Remarks : - Potential for human exposure - Inhalation route recommended for testing - Consider transgenic mouse model (p53 or TGAC) INPUT FROM GOVERNMENT AGENCIES/INDUSTRY Dr. -

Selective Hydrogenation of Cyclopentadiene to Form Cyclopentene
European Patent Office (n) Publication number: 0 009 035 Office europeen des brevets A1 EUROPEAN PATENT APPLICATION {ry Application number: 79930020.7 © lnt.CI.3: C 07 C 13/12 C 07 C 5/05, B 01 J 31/14 (22) Date of filing: 27.08.79 B 01 J 31/12 (30) Priority: 30.08.78 US 938159 (71) Applicant: THE GOODYEAR TIRE 8t RUBBER COMPANY 1144 East Market Street Akron, Ohio, 44316IUS) i*3) uaie ot puDiication ot application: 19.03.80 Bulletin 80 6 [73) Inventor: Menapace, Henry Robert 1738 Washington Circle @ Designated Contracting States: Stow Ohio 44224(US) BE DE FR GB IT NL g) Representative: Zewen, Arthur 4 Place Winston Churchill B.P. 447 Luxembourg-VillelLU ) {&) Selective hydrogenation of cyclopentadiene to form cyclopentene. where (57) Theremere is oiscloseddisclosed a process torfor the preparation otof cyc- wnere Hi,R1, H2R2 and R3 may bebe hydrogen or halogen, or an alkyl lopentene which comprises selectively hydrogenating cyc- radical containing from 1 to 6 carbon atoms; R,OR,R1OR2 wherein lopentadiene in a liquid phase by contacting cyclopentadiene R,Ri and R2 may bebe the same or different alkyl radicals contain- from with hydrogen in the presence of a hydrogenation catalyst inging 1 to 6 carbon atoms; and R^CR^ comprising (1(1) ) a soluble nickel compound, (2) an 0wherein wherein R,R1 may be organoaluminum compound or a lithium alkyl compound, alkylilkyl or an aromatic radical containing from 1 to 8 and (3) at least selected from the carbon one cocatalyst compound atomsitoms and RsR2 may be hydrogen or an alkyl or aromatic hyd- group consisting of HjO;H2O; NH3; ROH where R is an alkyl or a rocarbonocarbon radical containing from 1 to 8 carbon atoms. -

Secondary Organic Aerosol Formation from the Ozonolysis of Cycloalkenes and Related Compounds*
252 Chapter 7 Secondary Organic Aerosol Formation from the Ozonolysis of Cycloalkenes and Related Compounds* * This chapter is reproduced by permission from “Secondary organic aerosol formation from ozonolysis of cycloalkenes and related compounds” by M.D. Keywood, V. Varutbangkul, R. Bahreini, R.C. Flagan, J.H. Seinfeld, Environmental Science and Technology, 38 (15): 4157-4164, 2004. Copyright 2004, American Chemical Society. 253 7.1. Abstract The secondary organic aerosol (SOA) yields from the laboratory chamber ozonolysis of a series of cycloalkenes and related compounds are reported. The aim of this work is to investigate the effect of the structure of the hydrocarbon parent molecule on SOA formation for a homologous set of compounds. Aspects of the compound structures that are varied include the number of carbon atoms present in the cycloalkene ring (C5 to C8), the presence and location of methyl groups, and the presence of an exocyclic or endocyclic double bond. The specific compounds considered here are cyclopentene, cyclohexene, cycloheptene, cyclooctene, 1-methyl-1-cyclopentene, 1-methyl-1- cyclohexene, 1-methyl-1-cycloheptene, 3-methyl-1-cyclohexene, and methylene cyclohexane. SOA yield is found to be a function of the number of carbons present in the cycloalkene ring, with increasing number resulting in increased yield. Yield is enhanced by the presence of a methyl group located at a double-bonded site but reduced by the presence of a methyl group at a non-double bonded site. The presence of an exocyclic double bond also leads to a reduced yield relative to the equivalent methylated cycloalkene. On the basis of these observations, the SOA yield for terpinolene relative to the other cyclic alkenes is qualitatively predicted, and this prediction compares well to measurements of SOA yield from the ozonolysis of terpinolene. -

(12) United States Patent (10) Patent No.: US 7.494,962 B2 Kinet Al
USOO74949.62B2 (12) United States Patent (10) Patent No.: US 7.494,962 B2 Kinet al. (45) Date of Patent: Feb. 24, 2009 (54) SOLVENTS CONTAINING CYCLOAKYL (56) References Cited ALKYLETHERS AND PROCESS FOR PRODUCTION OF THE ETHERS U.S. PATENT DOCUMENTS 3496,223 A * 2/1970 Mitchell et al. ............... 562/22 (75) Inventors: Idan Kin, Ottawa (CA); Genichi Ohta, Tokyo (JP); Kazuo Teraishi, Tokyo (JP); Kiyoshi Watanabe, Tokyo (JP) (Continued) (73) Assignee: Zeon Corporation, Tokyo (JP) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this EP 587434 A1 3, 1994 patent is extended or adjusted under 35 U.S.C. 154(b) by 349 days. (Continued) (21) Appl. No.: 10/481,340 OTHER PUBLICATIONS (22) PCT Filed: Jun. 27, 2002 Edited by Kagaku Daijiten Henshu Iinkai, “Kagaku Daijiten 9”. (86). PCT No.: PCT/UP02/06501 Kyoritsu Shuppan Co., Ltd., Aug. 25, 1962, p. 437, "Yozai'. (Continued) S371 (c)(1), (2), (4) Date: Sep. 24, 2004 Primary Examiner Gregory E Webb (74) Attorney, Agent, or Firm—Birch, Stewart, Kolasch & (87) PCT Pub. No.: WO03/002500 Birch, LLP PCT Pub. Date: Jan. 9, 2003 (57) ABSTRACT (65) Prior Publication Data The present inventions are (A) a solvent comprising at least US 2005/OO65060A1 Mar. 24, 2005 one cycloalkyl alkyl ether (1) represented by the general O O formula: R1-O R2 (wherein R1 is cyclopentyl or the like: (30) Foreign Application Priority Data and R2 is C1-10 alkyl or the like); (B) a method of prepara Jun. 28, 2001 (JP) ............................. 2001-196766 tions the ethers (1) characterized by reacting an alicyclic Oct. -

Epoxidation of Cyclopentene, Cyclohexene and Cycloheptene by Acetylperoxyl Radicals
Epoxidation of Cyclopentene, Cyclohexene and Cycloheptene by Acetylperoxyl Radicals J. R. Lindsay Smith, D. M. S. Smith, M. S. Stark* and D. J. Waddington Department of Chemistry, University of York, York, YO10 5DD, United Kingdom To further the current understanding of how peroxyl radicals add to C=C double bonds,eg. 1-3 a series of cyclic alkenes (cyclopentene, cyclohexene and cycloheptene) were reacted with acetylperoxyl radicals over the temperature range 373 - 433 K, via the co- oxidation of acetaldehyde, the cyclic alkene and a reference alkene. The resulting epoxides were monitored by GC-FID, allowing the determination of the Arrhenius parameters given in Table 1 for these addition reactions. 3 -1 -1 -1 Alkene log10(A / dm mol s ) Eact / kJ mol cyclopentene 9.7±0.6 31.2±5.0 cyclohexene 7.7±0.7 17.4±5.3 cycloheptene 8.8±0.8 25.5±6.5 cis-2-butene4 8.1±0.5 22.9±3.8 Table 1: Arrhenius parameters for the addition of acetylperoxyl radicals to cyclic alkenes and cis-2-butene. Parameters for cis-2-butene are also given for comparison,4 as it would be expected that a relatively unstrained cycloalkene would behave in a similar manner to this non-cyclic alkene; this is indeed found to be so, with no statistically significant difference between cyclohexene and cis-2-butene. Indeed the measured activation energy for cyclohexene fits in well with correlation between charge transfer ( Ec) and activation energy that is known to exist for the addition of acetylperoxyl to acyclic mono-alkenes.1 Cyclopentene however does have a significantly larger pre-exponential factor and activation energy than either cyclohexene or cis-2-butene. -

A Comparative Study of the Formation of Aromatics In
A COMPARATIVE STUDY OF THE FORMATION OF AROMATICS IN RICH METHANE FLAMES DOPED BY UNSATURATED COMPOUNDS H.A. GUENICHE, J. BIET, P.A. GLAUDE, R. FOURNET, F. BATTIN-LECLERC* Département de Chimie-Physique des Réactions, Nancy Université, CNRS, 1 rue Grandville, BP 20451, 54001 NANCY Cedex, France * E-mail : [email protected] ; Tel.: 33 3 83 17 51 25 , Fax : 33 3 83 37 81 20 ABSTRACT For a better modeling of the importance of the different channels leading to the first aromatic ring, we have compared the structures of laminar rich premixed methane flames doped with several unsaturated hydrocarbons: allene and propyne, because they are precursors of propargyl radicals which are well known as having an important role in forming benzene, 1,3-butadiene to put in evidence a possible production of benzene due to reactions of C4 compounds, and, finally, cyclopentene which is a source of cyclopentadienylmethylene radicals which in turn are expected to easily isomerizes to give benzene. These flames have been stabilized on a burner at a pressure of 6.7 kPa (50 Torr) using argon as dilutant, for equivalence ratios (φ) from 1.55 to 1.79. A unique mechanism, including the formation and decomposition of benzene and toluene, has been used to model the oxidation of allene, propyne, 1,3-butadiene and cyclopentene. The main reaction pathways of aromatics formation have been derived from reaction rate and sensitivity analyses and have been compared for the three types of additives. These combined analyses and comparisons can only been performed when a unique mechanism is available for all the studied additives. -

The Arene–Alkene Photocycloaddition
The arene–alkene photocycloaddition Ursula Streit and Christian G. Bochet* Review Open Access Address: Beilstein J. Org. Chem. 2011, 7, 525–542. Department of Chemistry, University of Fribourg, Chemin du Musée 9, doi:10.3762/bjoc.7.61 CH-1700 Fribourg, Switzerland Received: 07 January 2011 Email: Accepted: 23 March 2011 Ursula Streit - [email protected]; Christian G. Bochet* - Published: 28 April 2011 [email protected] This article is part of the Thematic Series "Photocycloadditions and * Corresponding author photorearrangements". Keywords: Guest Editor: A. G. Griesbeck benzene derivatives; cycloadditions; Diels–Alder; photochemistry © 2011 Streit and Bochet; licensee Beilstein-Institut. License and terms: see end of document. Abstract In the presence of an alkene, three different modes of photocycloaddition with benzene derivatives can occur; the [2 + 2] or ortho, the [3 + 2] or meta, and the [4 + 2] or para photocycloaddition. This short review aims to demonstrate the synthetic power of these photocycloadditions. Introduction Photocycloadditions occur in a variety of modes [1]. The best In the presence of an alkene, three different modes of photo- known representatives are undoubtedly the [2 + 2] photocyclo- cycloaddition with benzene derivatives can occur, viz. the addition, forming either cyclobutanes or four-membered hetero- [2 + 2] or ortho, the [3 + 2] or meta, and the [4 + 2] or para cycles (as in the Paternò–Büchi reaction), whilst excited-state photocycloaddition (Scheme 2). The descriptors ortho, meta [4 + 4] cycloadditions can also occur to afford cyclooctadiene and para only indicate the connectivity to the aromatic ring, and compounds. On the other hand, the well-known thermal [4 + 2] do not have any implication with regard to the reaction mecha- cycloaddition (Diels–Alder reaction) is only very rarely nism. -

Guidance Document Peroxide-Forming Chemicals
Guidance Document Peroxide-Forming Chemicals Some chemicals can form peroxides under normal storage conditions. Some of the peroxide chemicals are unstable, especially when dried or concentrated, and can explode violently when subjected to heat, light or mechanical shock. In addition, some of the inadvertently formed peroxides can initiate other unexpected violent reactions (e.g. polymerizations) with other chemicals. When possible and practical for your work, purchase chemicals that have inhibitors added by the manufacturer. Label peroxide-forming chemicals with date received and date opened. Store peroxide-formers in airtight opaque containers with screw caps. Consider oxygen exclusion methods such as purging with inert gas or sealing containers with parafilm. Inspect containers for signs of peroxide formation. Do not open a container which has crystals or a visible cloudiness. Call EHS to come remove it. The friction caused by opening a lid can cause an explosion. Liquids can be tested for presence of peroxide. This is especially important prior to distilation. Most explosions of peroxide forming chemicals occur when a material is distilled to dryness. Peroxide test kits are available from chemical vendors. Contact EHS for additional guidance. Classification Table for Peroxide-Forming Chemicals Class I:: Unsaturated materials, especially those of low molecular weight, may polymerize violently and hazardously due to peroxide initiation. These chemicals can spontaneously decompose, becoming explosive after exposure to air with concentration. Discard unopened containers within 3 months. Opened containers should be tested for peroxides every 2 months. Acrylic acid Tetrafluoroethylene Acrylonitrile Vinyl acetate 1,3-Butadiene Vinyl acetylene Chlorobutadiene (chloroprene) Vinyl chloride Chlorotrifluoroethylene Vinyl pyridine Methyl methacrylate Vinylidiene chloride Styrene Class II: The following chemicals are a peroxide hazard upon concentration (distillation/evaporation). -

NMR Chemical Shifts of Common Laboratory Solvents As Trace Impurities
7512 J. Org. Chem. 1997, 62, 7512-7515 NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities Hugo E. Gottlieb,* Vadim Kotlyar, and Abraham Nudelman* Department of Chemistry, Bar-Ilan University, Ramat-Gan 52900, Israel Received June 27, 1997 In the course of the routine use of NMR as an aid for organic chemistry, a day-to-day problem is the identifica- tion of signals deriving from common contaminants (water, solvents, stabilizers, oils) in less-than-analyti- cally-pure samples. This data may be available in the literature, but the time involved in searching for it may be considerable. Another issue is the concentration dependence of chemical shifts (especially 1H); results obtained two or three decades ago usually refer to much Figure 1. Chemical shift of HDO as a function of tempera- more concentrated samples, and run at lower magnetic ture. fields, than today’s practice. 1 13 We therefore decided to collect H and C chemical dependent (vide infra). Also, any potential hydrogen- shifts of what are, in our experience, the most popular bond acceptor will tend to shift the water signal down- “extra peaks” in a variety of commonly used NMR field; this is particularly true for nonpolar solvents. In solvents, in the hope that this will be of assistance to contrast, in e.g. DMSO the water is already strongly the practicing chemist. hydrogen-bonded to the solvent, and solutes have only a negligible effect on its chemical shift. This is also true Experimental Section for D2O; the chemical shift of the residual HDO is very NMR spectra were taken in a Bruker DPX-300 instrument temperature-dependent (vide infra) but, maybe counter- (300.1 and 75.5 MHz for 1H and 13C, respectively). -
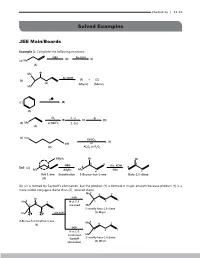
11.Alkenes-And-Alkynesexercise.Pdf
Chemistry | 11.43 Solved Examples JEE Main/Boards Example 1: Complete the following reactions: NBS Alc.KOH (B) (C ) (a) Me (A) Me Br Alc.KOH (b) (Y) + (Z) (X) (Major) (Minor) Me H (c ) (B) (A) Cl 1. Li -B 2 (B) (C ) (D) (d) Me at 500oC 2. CuI (A) (e) HO KHSO 4 (Y) OH or (X) Al23O or PO25 Allylic Br Br 3 1 3 NBS Alc. KOH Sol: (a) 4 4 Me Allylic Me 2 -Hbr 2 But-1-ene Substitution 3-Bromo-but-1-ene Buta-1,3-diene (A) (b) (Z) is formed by Saytzeff’s elimination, but the product (Y) is formed in major amount because product (Y) is a more stable conjugate diene than (Z), isolated diene 6 Me 4 2 -HBr Br 1 Me6 H at C-3 5 3 4 2 removed Me (b) 5 3 1 5-methly hexa-1,3-diene Me H H Alk.KOH (Y) Major 4-Bromo-5-methylhex-1-ene Me6 (X) 4 2 -HBr 5 1 H at C-3 3 is removed Me Saytzeff 5-methly hexa-1,4-diene elimination (Z) Minor 11.44 | Alkenes and Alkynes (c) 4 1 H H 3 5 -H 2 6 ( c ) H H 2 6 3 5 1 4 Less stable More stable isolated diene conjugated diene (A) (B) Cyclohexa-1,4-diene Cyclohexa-1,3-diene 2 o 2 Cl at 500 2 4 2 3 1. Li (d) 1 1 1 Me Allylic 2. Cu 3 (d) Prop-1-ene substitution Cl Corey-House Allylic synthesis Diallyl R (A) 3-Chloro- lithium cuperate prop-1-ene (B) Cl 4 4 6 1 3 5 R’ H (e) (e) HO 2 4 KHSO4 OH Or 1 3 H Al23O or PO25 (X) (Y) (-H2O) Butan-1,4-diol Butan-1,3-diene Example 2: Give the major products (not stereoisomers) of the following: 5 5 (a 1 4 (a)(a) 1 4 22 33 Cyclopenta-1,3-diene 1 1mo mol l 1 mo1 mol l 1 mo1l mol 11 mol of BrBr22 HBrHBr + + HBHBr r HS2 +HS + peroxide 2 peroxide peroperoxide xide (b) 55 (b) 1 (b) Me 1 4 Me 4 2 3 3 1-Ethylcyclopenta-1,3-diene2 1-Ethylcyclopenta-1,3-diene 1 mol 1 mol 1 mol 1 mol of Br2 1 mol of Br HBr1 mo + l HBr1 mol 1 mol 2 HS2 + peroHBrxide + HBr peroHSxide2 + peroxide peroxide Chemistry | 11.45 Sol: 1 (a) (a) 2 5 3 4 (A) All 1,4-addition products HBr or HS+ Br2 2 1,4-Add. -

Contribution of Cyclopentyl Methyl Ether (CPME) to Green Chemistry
sustainability / green chemistry Industry perspective Shunji Sakamoto Contribution of Cyclopentyl Methyl Ether (CPME) to Green Chemistry Shunji sakamoto Specialty Chemicals Division, ZEON CORPORATION, 1-6-2 Marunouchi, Chiyoda-ku, Tokyo, 1008246, Japan easy peroxide formation and water miscibility, the demand KEyWORDS for CPME has steadily been growing in the pharmaceutical Cyclopentyl methyl ether, CPME, Green Chemistry, industry as well as in the electronic and fragrance solvent, process, ICH. industries. Also, CPME has been registered or listed in the corresponding legislations for new chemical substances of United States of America, Canada, European Union, ABSTRACT Japan, South Korea, China and Chinese Taipei, and is commercially available in these countries and regions as Cyclopentyl methyl ether (CPME) is an alternative well as others including India. solvent and has preferable properties including higher In this article, I answer the question why CPME is “Green” hydrophobicity, lower formation of peroxides and better even though it is derived from petroleum. stability under acidic and basic conditions compared to other traditional ether solvents such as tetrahydrofuran. Due to the above unique properties, CPME has particular UNIQUE FEATURES OF CPME 24 advantages and renders some conventional reaction sequences in one pot or easier process as a result of CPME is an asymmetric aliphatic ether having a cyclopentyl solvent unification and facile isolation of the products, group. It has seven unique features attributed to its structure which contribute to Green Chemistry as well as to (Table 1). process innovation. CPME meets eight definitions out of the 12 Principles of Green Chemistry (1) because of Extremely lower miscibility with water the above characteristics as well as its manufacturing The solubility of CPME in water is only 1.1% and that of water process and its applications. -

Q3C (R8): Impurities: Guideline for Residual Solvents Step 2B
04 May 2020 EMA/CHMP/ICH/213867/2020 Committee for Medicinal Products for Human Use Q3C (R8): Impurities: guideline for residual solvents Step 2b Transmission to CHMP 30 April 2020 Adoption by CHMP 30 April 2020 Release for public consultation 4 May 2020 Deadline for comments 30 July 2020 Comments should be provided using this template. The completed comments form should be sent to [email protected] Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000An agency of the European Union © European Medicines Agency, 2020. Reproduction is authorised provided the source is acknowledged. PDE for 2-Methyltetrahydrofuran, Cyclopentyl Methyl Ether, and Tertiary-Butyl Alcohol INTERNATIONAL COUNCIL FOR HARMONISATION OF TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED GUIDELINE IMPURITIES: GUIDELINE FOR RESIDUAL SOLVENTS Q3C(R8) PDE FOR 2-METHYLTETRAHYDROFURAN, CYCLOPENTYL METHYL ETHER, AND TERTIARY-BUTYL ALCOHOL Draft version Endorsed on 25 March 2020 Currently under public consultation Note : This document contains only the PDE levels for three solvents: 2- methyltetrahydrofuran, cyclopentylmethylether and tert-butanol that were agreed to be included in the ICH Q3C(R8) revision. Further to reaching Step 4, these PDEs would be integrated into a complete Q3C(R8) Guideline document. At Step 2 of the ICH Process, a consensus draft text or guideline, agreed by the appropriate ICH Expert Working Group, is transmitted by the ICH Assembly to the regulatory authorities PDE for 2-Methyltetrahydrofuran, Cyclopentyl Methyl Ether, and Tertiary-Butyl Alcohol of the ICH regions for internal and external consultation, according to national or regional procedures.