Epoxidation of Cyclopentene, Cyclohexene and Cycloheptene by Acetylperoxyl Radicals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Measuring and Predicting Sooting Tendencies of Oxygenates, Alkanes, Alkenes, Cycloalkanes, and Aromatics on a Unified Scale
Measuring and Predicting Sooting Tendencies of Oxygenates, Alkanes, Alkenes, Cycloalkanes, and Aromatics on a Unified Scale Dhrubajyoti D. Dasa,1, Peter St. Johnb,1, Charles S. McEnallya,∗, Seonah Kimb, Lisa D. Pfefferlea aYale University, Department of Chemical and Environmental Engineering, New Haven CT 06520 bNational Renewable Energy Laboratory, Golden CO 80401 Abstract Soot from internal combustion engines negatively affects health and climate. Soot emissions might be reduced through the expanded usage of appropriate biomass-derived fuels. Databases of sooting indices, based on measuring some aspect of sooting behavior in a standardized combustion environment, are useful in providing information on the comparative sooting tendencies of different fuels or pure compounds. However, newer biofuels have varied chemical structures including both aromatic and oxygenated functional groups, making an accurate measurement or prediction of their sooting tendency difficult. In this work, we propose a unified sooting tendency database for pure compounds, including both regular and oxygenated hydrocarbons, which is based on combining two disparate databases of yield-based sooting tendency measurements in the literature. Unification of the different databases was made possible by leveraging the greater dynamic range of the color ratio pyrometry soot diagnostic. This unified database contains a substantial number of pure compounds (≥ 400 total) from multiple categories of hydrocarbons important in modern fuels and establishes the sooting tendencies of aromatic and oxygenated hydrocarbons on the same numeric scale for the first time. Using this unified sooting tendency database, we have developed a predictive model for sooting behavior applicable to a broad range of hydrocarbons and oxygenated hydrocarbons. The model decomposes each compound into single-carbon fragments and assigns a sooting tendency contribution to each fragment based on regression against the unified database. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Selective Hydrogenation of Cyclopentadiene to Form Cyclopentene
European Patent Office (n) Publication number: 0 009 035 Office europeen des brevets A1 EUROPEAN PATENT APPLICATION {ry Application number: 79930020.7 © lnt.CI.3: C 07 C 13/12 C 07 C 5/05, B 01 J 31/14 (22) Date of filing: 27.08.79 B 01 J 31/12 (30) Priority: 30.08.78 US 938159 (71) Applicant: THE GOODYEAR TIRE 8t RUBBER COMPANY 1144 East Market Street Akron, Ohio, 44316IUS) i*3) uaie ot puDiication ot application: 19.03.80 Bulletin 80 6 [73) Inventor: Menapace, Henry Robert 1738 Washington Circle @ Designated Contracting States: Stow Ohio 44224(US) BE DE FR GB IT NL g) Representative: Zewen, Arthur 4 Place Winston Churchill B.P. 447 Luxembourg-VillelLU ) {&) Selective hydrogenation of cyclopentadiene to form cyclopentene. where (57) Theremere is oiscloseddisclosed a process torfor the preparation otof cyc- wnere Hi,R1, H2R2 and R3 may bebe hydrogen or halogen, or an alkyl lopentene which comprises selectively hydrogenating cyc- radical containing from 1 to 6 carbon atoms; R,OR,R1OR2 wherein lopentadiene in a liquid phase by contacting cyclopentadiene R,Ri and R2 may bebe the same or different alkyl radicals contain- from with hydrogen in the presence of a hydrogenation catalyst inging 1 to 6 carbon atoms; and R^CR^ comprising (1(1) ) a soluble nickel compound, (2) an 0wherein wherein R,R1 may be organoaluminum compound or a lithium alkyl compound, alkylilkyl or an aromatic radical containing from 1 to 8 and (3) at least selected from the carbon one cocatalyst compound atomsitoms and RsR2 may be hydrogen or an alkyl or aromatic hyd- group consisting of HjO;H2O; NH3; ROH where R is an alkyl or a rocarbonocarbon radical containing from 1 to 8 carbon atoms. -

Cyclopentyl Methyl Ether (CPME)
Korean J. Chem. Eng., 34(2), 463-469 (2017) pISSN: 0256-1115 DOI: 10.1007/s11814-016-0265-5 eISSN: 1975-7220 INVITED REVIEW PAPER Measurement and correlation of the isothermal VLE data for the binary mixtures of cyclopentene (CPEN)+cyclopentyl methyl ether (CPME) Wan Ju Jeong and Jong Sung Lim† Department of Chemical and Biomolecular Engineering, Sogang University, C.P.O. Box 1142, Seoul 04107, Korea (Received 2 August 2016 • accepted 20 September 2016) Abstract−The isothermal vapor-liquid equilibrium data for the binary systems of cyclopentene (1)+cyclopentyl methyl ether (2) were measured at 313.15, 323.15, 333.15, 343.15 and 353.15 K using a dynamic-type equilibrium apparatus and online gas chromatography analysis. For all the measured VLE data consistency tests were performed for the verifi- cation of data using Barker’s method and the ASPEN PLUS Area Test method. All the resulting average absolute val- δ γ γ ues of residuals [ ln ( 1/ 2)] for Barker’s method and D values for the ASPEN PLUS area test method were com- paratively small. So, the VLE data reported in this study are considered to be acceptable. This binary system shows neg- ative deviation from Raoult’s law and does not exhibit azeotropic behavior at whole temperature ranges studied here. The measured data were correlated with the P-R EoS using the Wong-Sandler mixing rule. The overall average relative deviation of pressure (ARD-P (%)) between experimental and calculated values was 0.078% and that of vapor phase compositions (ARD-y (%)) was 0.452%. Keywords: Vapor Liquid Equilibria (VLE), Cyclopentyl Methyl Ether (CPME), Cyclopentene (CPEN), Peng-Robinson Equation of State (PR-EoS), Wong-Sandler Mixing Rule (WS-MR) INTRODUCTION however, have some disadvantages because they use dimethyl sul- fate and methyl iodide as a reactant, respectively, which are muta- Ethers are widely used in organic chemistry and biochemistry. -

Secondary Organic Aerosol Formation from the Ozonolysis of Cycloalkenes and Related Compounds*
252 Chapter 7 Secondary Organic Aerosol Formation from the Ozonolysis of Cycloalkenes and Related Compounds* * This chapter is reproduced by permission from “Secondary organic aerosol formation from ozonolysis of cycloalkenes and related compounds” by M.D. Keywood, V. Varutbangkul, R. Bahreini, R.C. Flagan, J.H. Seinfeld, Environmental Science and Technology, 38 (15): 4157-4164, 2004. Copyright 2004, American Chemical Society. 253 7.1. Abstract The secondary organic aerosol (SOA) yields from the laboratory chamber ozonolysis of a series of cycloalkenes and related compounds are reported. The aim of this work is to investigate the effect of the structure of the hydrocarbon parent molecule on SOA formation for a homologous set of compounds. Aspects of the compound structures that are varied include the number of carbon atoms present in the cycloalkene ring (C5 to C8), the presence and location of methyl groups, and the presence of an exocyclic or endocyclic double bond. The specific compounds considered here are cyclopentene, cyclohexene, cycloheptene, cyclooctene, 1-methyl-1-cyclopentene, 1-methyl-1- cyclohexene, 1-methyl-1-cycloheptene, 3-methyl-1-cyclohexene, and methylene cyclohexane. SOA yield is found to be a function of the number of carbons present in the cycloalkene ring, with increasing number resulting in increased yield. Yield is enhanced by the presence of a methyl group located at a double-bonded site but reduced by the presence of a methyl group at a non-double bonded site. The presence of an exocyclic double bond also leads to a reduced yield relative to the equivalent methylated cycloalkene. On the basis of these observations, the SOA yield for terpinolene relative to the other cyclic alkenes is qualitatively predicted, and this prediction compares well to measurements of SOA yield from the ozonolysis of terpinolene. -

(12) United States Patent (10) Patent No.: US 7.494,962 B2 Kinet Al
USOO74949.62B2 (12) United States Patent (10) Patent No.: US 7.494,962 B2 Kinet al. (45) Date of Patent: Feb. 24, 2009 (54) SOLVENTS CONTAINING CYCLOAKYL (56) References Cited ALKYLETHERS AND PROCESS FOR PRODUCTION OF THE ETHERS U.S. PATENT DOCUMENTS 3496,223 A * 2/1970 Mitchell et al. ............... 562/22 (75) Inventors: Idan Kin, Ottawa (CA); Genichi Ohta, Tokyo (JP); Kazuo Teraishi, Tokyo (JP); Kiyoshi Watanabe, Tokyo (JP) (Continued) (73) Assignee: Zeon Corporation, Tokyo (JP) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this EP 587434 A1 3, 1994 patent is extended or adjusted under 35 U.S.C. 154(b) by 349 days. (Continued) (21) Appl. No.: 10/481,340 OTHER PUBLICATIONS (22) PCT Filed: Jun. 27, 2002 Edited by Kagaku Daijiten Henshu Iinkai, “Kagaku Daijiten 9”. (86). PCT No.: PCT/UP02/06501 Kyoritsu Shuppan Co., Ltd., Aug. 25, 1962, p. 437, "Yozai'. (Continued) S371 (c)(1), (2), (4) Date: Sep. 24, 2004 Primary Examiner Gregory E Webb (74) Attorney, Agent, or Firm—Birch, Stewart, Kolasch & (87) PCT Pub. No.: WO03/002500 Birch, LLP PCT Pub. Date: Jan. 9, 2003 (57) ABSTRACT (65) Prior Publication Data The present inventions are (A) a solvent comprising at least US 2005/OO65060A1 Mar. 24, 2005 one cycloalkyl alkyl ether (1) represented by the general O O formula: R1-O R2 (wherein R1 is cyclopentyl or the like: (30) Foreign Application Priority Data and R2 is C1-10 alkyl or the like); (B) a method of prepara Jun. 28, 2001 (JP) ............................. 2001-196766 tions the ethers (1) characterized by reacting an alicyclic Oct. -

A Comparative Study of the Formation of Aromatics In
A COMPARATIVE STUDY OF THE FORMATION OF AROMATICS IN RICH METHANE FLAMES DOPED BY UNSATURATED COMPOUNDS H.A. GUENICHE, J. BIET, P.A. GLAUDE, R. FOURNET, F. BATTIN-LECLERC* Département de Chimie-Physique des Réactions, Nancy Université, CNRS, 1 rue Grandville, BP 20451, 54001 NANCY Cedex, France * E-mail : [email protected] ; Tel.: 33 3 83 17 51 25 , Fax : 33 3 83 37 81 20 ABSTRACT For a better modeling of the importance of the different channels leading to the first aromatic ring, we have compared the structures of laminar rich premixed methane flames doped with several unsaturated hydrocarbons: allene and propyne, because they are precursors of propargyl radicals which are well known as having an important role in forming benzene, 1,3-butadiene to put in evidence a possible production of benzene due to reactions of C4 compounds, and, finally, cyclopentene which is a source of cyclopentadienylmethylene radicals which in turn are expected to easily isomerizes to give benzene. These flames have been stabilized on a burner at a pressure of 6.7 kPa (50 Torr) using argon as dilutant, for equivalence ratios (φ) from 1.55 to 1.79. A unique mechanism, including the formation and decomposition of benzene and toluene, has been used to model the oxidation of allene, propyne, 1,3-butadiene and cyclopentene. The main reaction pathways of aromatics formation have been derived from reaction rate and sensitivity analyses and have been compared for the three types of additives. These combined analyses and comparisons can only been performed when a unique mechanism is available for all the studied additives. -

The Arene–Alkene Photocycloaddition
The arene–alkene photocycloaddition Ursula Streit and Christian G. Bochet* Review Open Access Address: Beilstein J. Org. Chem. 2011, 7, 525–542. Department of Chemistry, University of Fribourg, Chemin du Musée 9, doi:10.3762/bjoc.7.61 CH-1700 Fribourg, Switzerland Received: 07 January 2011 Email: Accepted: 23 March 2011 Ursula Streit - [email protected]; Christian G. Bochet* - Published: 28 April 2011 [email protected] This article is part of the Thematic Series "Photocycloadditions and * Corresponding author photorearrangements". Keywords: Guest Editor: A. G. Griesbeck benzene derivatives; cycloadditions; Diels–Alder; photochemistry © 2011 Streit and Bochet; licensee Beilstein-Institut. License and terms: see end of document. Abstract In the presence of an alkene, three different modes of photocycloaddition with benzene derivatives can occur; the [2 + 2] or ortho, the [3 + 2] or meta, and the [4 + 2] or para photocycloaddition. This short review aims to demonstrate the synthetic power of these photocycloadditions. Introduction Photocycloadditions occur in a variety of modes [1]. The best In the presence of an alkene, three different modes of photo- known representatives are undoubtedly the [2 + 2] photocyclo- cycloaddition with benzene derivatives can occur, viz. the addition, forming either cyclobutanes or four-membered hetero- [2 + 2] or ortho, the [3 + 2] or meta, and the [4 + 2] or para cycles (as in the Paternò–Büchi reaction), whilst excited-state photocycloaddition (Scheme 2). The descriptors ortho, meta [4 + 4] cycloadditions can also occur to afford cyclooctadiene and para only indicate the connectivity to the aromatic ring, and compounds. On the other hand, the well-known thermal [4 + 2] do not have any implication with regard to the reaction mecha- cycloaddition (Diels–Alder reaction) is only very rarely nism. -

Cycloalkanes, Cycloalkenes, and Cycloalkynes
CYCLOALKANES, CYCLOALKENES, AND CYCLOALKYNES any important hydrocarbons, known as cycloalkanes, contain rings of carbon atoms linked together by single bonds. The simple cycloalkanes of formula (CH,), make up a particularly important homologous series in which the chemical properties change in a much more dramatic way with increasing n than do those of the acyclic hydrocarbons CH,(CH,),,-,H. The cyclo- alkanes with small rings (n = 3-6) are of special interest in exhibiting chemical properties intermediate between those of alkanes and alkenes. In this chapter we will show how this behavior can be explained in terms of angle strain and steric hindrance, concepts that have been introduced previously and will be used with increasing frequency as we proceed further. We also discuss the conformations of cycloalkanes, especially cyclo- hexane, in detail because of their importance to the chemistry of many kinds of naturally occurring organic compounds. Some attention also will be paid to polycyclic compounds, substances with more than one ring, and to cyclo- alkenes and cycloalkynes. 12-1 NOMENCLATURE AND PHYSICAL PROPERTIES OF CYCLOALKANES The IUPAC system for naming cycloalkanes and cycloalkenes was presented in some detail in Sections 3-2 and 3-3, and you may wish to review that ma- terial before proceeding further. Additional procedures are required for naming 446 12 Cycloalkanes, Cycloalkenes, and Cycloalkynes Table 12-1 Physical Properties of Alkanes and Cycloalkanes Density, Compounds Bp, "C Mp, "C diO,g ml-' propane cyclopropane butane cyclobutane pentane cyclopentane hexane cyclohexane heptane cycloheptane octane cyclooctane nonane cyclononane "At -40". bUnder pressure. polycyclic compounds, which have rings with common carbons, and these will be discussed later in this chapter. -
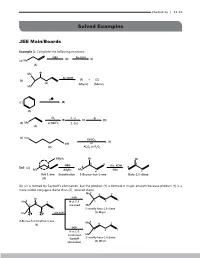
11.Alkenes-And-Alkynesexercise.Pdf
Chemistry | 11.43 Solved Examples JEE Main/Boards Example 1: Complete the following reactions: NBS Alc.KOH (B) (C ) (a) Me (A) Me Br Alc.KOH (b) (Y) + (Z) (X) (Major) (Minor) Me H (c ) (B) (A) Cl 1. Li -B 2 (B) (C ) (D) (d) Me at 500oC 2. CuI (A) (e) HO KHSO 4 (Y) OH or (X) Al23O or PO25 Allylic Br Br 3 1 3 NBS Alc. KOH Sol: (a) 4 4 Me Allylic Me 2 -Hbr 2 But-1-ene Substitution 3-Bromo-but-1-ene Buta-1,3-diene (A) (b) (Z) is formed by Saytzeff’s elimination, but the product (Y) is formed in major amount because product (Y) is a more stable conjugate diene than (Z), isolated diene 6 Me 4 2 -HBr Br 1 Me6 H at C-3 5 3 4 2 removed Me (b) 5 3 1 5-methly hexa-1,3-diene Me H H Alk.KOH (Y) Major 4-Bromo-5-methylhex-1-ene Me6 (X) 4 2 -HBr 5 1 H at C-3 3 is removed Me Saytzeff 5-methly hexa-1,4-diene elimination (Z) Minor 11.44 | Alkenes and Alkynes (c) 4 1 H H 3 5 -H 2 6 ( c ) H H 2 6 3 5 1 4 Less stable More stable isolated diene conjugated diene (A) (B) Cyclohexa-1,4-diene Cyclohexa-1,3-diene 2 o 2 Cl at 500 2 4 2 3 1. Li (d) 1 1 1 Me Allylic 2. Cu 3 (d) Prop-1-ene substitution Cl Corey-House Allylic synthesis Diallyl R (A) 3-Chloro- lithium cuperate prop-1-ene (B) Cl 4 4 6 1 3 5 R’ H (e) (e) HO 2 4 KHSO4 OH Or 1 3 H Al23O or PO25 (X) (Y) (-H2O) Butan-1,4-diol Butan-1,3-diene Example 2: Give the major products (not stereoisomers) of the following: 5 5 (a 1 4 (a)(a) 1 4 22 33 Cyclopenta-1,3-diene 1 1mo mol l 1 mo1 mol l 1 mo1l mol 11 mol of BrBr22 HBrHBr + + HBHBr r HS2 +HS + peroxide 2 peroxide peroperoxide xide (b) 55 (b) 1 (b) Me 1 4 Me 4 2 3 3 1-Ethylcyclopenta-1,3-diene2 1-Ethylcyclopenta-1,3-diene 1 mol 1 mol 1 mol 1 mol of Br2 1 mol of Br HBr1 mo + l HBr1 mol 1 mol 2 HS2 + peroHBrxide + HBr peroHSxide2 + peroxide peroxide Chemistry | 11.45 Sol: 1 (a) (a) 2 5 3 4 (A) All 1,4-addition products HBr or HS+ Br2 2 1,4-Add. -

Alkenes and Alkynes
02/21/2019 CHAPTER FOUR Alkenes and Alkynes H N O I Cl C O C O Cl F3C C Cl C Cl Efavirenz Haloprogin (antiviral, AIDS therapeutic) (antifungal, antiseptic) Chapter 4 Table of Content * Unsaturated Hydrocarbons * Introduction and hybridization * Alkenes and Alkynes * Benzene and Phenyl groups * Structure of Alkenes, cis‐trans Isomerism * Nomenclature of Alkenes and Alkynes * Configuration cis/trans, and cis/trans Isomerism * Configuration E/Z * Physical Properties of Hydrocarbons * Acid‐Base Reactions of Hydrocarbons * pka and Hybridizations 1 02/21/2019 Unsaturated Hydrocarbons • Unsaturated Hydrocarbon: A hydrocarbon that contains one or more carbon‐carbon double or triple bonds or benzene‐like rings. – Alkene: contains a carbon‐carbon double bond and has the general formula CnH2n. – Alkyne: contains a carbon‐carbon triple bond and has the general formula CnH2n‐2. Introduction Alkenes ● Hydrocarbons containing C=C ● Old name: olefins • Steroids • Hormones • Biochemical regulators 2 02/21/2019 • Alkynes – Hydrocarbons containing C≡C – Common name: acetylenes Unsaturated Hydrocarbons • Arene: benzene and its derivatives (Ch 9) 3 02/21/2019 Benzene and Phenyl Groups • We do not study benzene and its derivatives until Chapter 9. – However, we show structural formulas of compounds containing a phenyl group before that time. – The phenyl group is not reactive under any of the conditions we describe in chapters 5‐8. Structure of Alkenes • The two carbon atoms of a double bond and the four atoms bonded to them lie in a plane, with bond angles of approximately 120°. 4 02/21/2019 Structure of Alkenes • Figure 4.1 According to the orbital overlap model, a double bond consists of one bond formed by overlap of sp2 hybrid orbitals and one bond formed by overlap of parallel 2p orbitals. -

Spatial Variability of Air Pollutants in a Megacity Characterized by Mobile Measurements: Chemical Homogeneity Under Haze Conditions
Supplementary Material for Spatial variability of air pollutants in a megacity characterized by mobile measurements: Chemical homogeneity under haze conditions Reza Bashiri Khuzestani1,a,★, Keren Liao1,★, Qi Chen1,*, Ying Liu1, Yan Zheng1, Xi Cheng1, Tianjiao Jia1, Xin Li1, Shiyi Chen1, Guancong Huang1 1State Key Joint Laboratory of Environmental Simulation and Pollution Control, BIC-ESAT and IJRC, College of Environmental Sciences and Engineering, Peking University, Beijing, 100871, China aNow at: Faculty of Civil, Water and Environmental Engineering, School of Engineering, Shahid Beheshti University, Tehran, Iran. ★These authors contributed equally to this work. *Correspondence to: Qi Chen ([email protected]) Table S1. The average concentrations of the 52 VOC species measured in this study compared with literature. These species are tentatively categorized into three groups including hydrocarbons (Group 1), aldehydes and ketones (Group 2), and acids and anhydrides (Group 3). Category Mean ± sd Mean ± sd * * Formula Assigned Name m/z KPTR Urban Suburb /Group (non-haze) (haze) + (CH3OH)H Methanol 33.033 2.22 n/a 16.82±14.81 44.69±15.96 11.77-51.76 3.4-5.6 + (C2H2O)H Ketenes 43.018 2.21 2 2.12±1.84 5.42±1.42 + (C2H4O)H Acetaldehyde 45.033 3.36 2 2.72±2.00 6.07±1.63 1.88-15.81 0.83-1.23 + (C2H6O)H Ethanol 47.049 2.18 n/a 25.35±32.19 98.28±32.17 + (C3H4O)H Acrolein; MTBE 57.033 3.35 2 0.30±0.27 0.60±0.14 (C H O)H+ Acetone + propanal 59.049 3 2 1.20±1.18 4.67±0.97 2.48-7.92 1.59-3.42 3 6 Isoprene; fragmentation of 2- methyl-3- + (C5H8)H buten-2-ol (MBO); fragmentation of 69.07 1.94 1 0.38±0.35 0.76±0.20 cyclohexanes species Methyl vinyl ketone + methacrolein; ted + (C4H6O)H 71.049 3.83 2 0.16±0.14 0.36±0.09 0.28-0.42 0.13-0.22 crotonaldehyde; ISOPOOH + (C4H8O)H Methyl ethyl ketone + butanals 73.065 3.48 2 1.56±2.45 1.51±0.44 0.86-2.53 0.38-0.81 Calibra + (C6H6)H Benzene 79.054 1.97 1 0.