Microgale Jobihely) from Northern Madagascar S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecosystem Profile Madagascar and Indian
ECOSYSTEM PROFILE MADAGASCAR AND INDIAN OCEAN ISLANDS FINAL VERSION DECEMBER 2014 This version of the Ecosystem Profile, based on the draft approved by the Donor Council of CEPF was finalized in December 2014 to include clearer maps and correct minor errors in Chapter 12 and Annexes Page i Prepared by: Conservation International - Madagascar Under the supervision of: Pierre Carret (CEPF) With technical support from: Moore Center for Science and Oceans - Conservation International Missouri Botanical Garden And support from the Regional Advisory Committee Léon Rajaobelina, Conservation International - Madagascar Richard Hughes, WWF – Western Indian Ocean Edmond Roger, Université d‘Antananarivo, Département de Biologie et Ecologie Végétales Christopher Holmes, WCS – Wildlife Conservation Society Steve Goodman, Vahatra Will Turner, Moore Center for Science and Oceans, Conservation International Ali Mohamed Soilihi, Point focal du FEM, Comores Xavier Luc Duval, Point focal du FEM, Maurice Maurice Loustau-Lalanne, Point focal du FEM, Seychelles Edmée Ralalaharisoa, Point focal du FEM, Madagascar Vikash Tatayah, Mauritian Wildlife Foundation Nirmal Jivan Shah, Nature Seychelles Andry Ralamboson Andriamanga, Alliance Voahary Gasy Idaroussi Hamadi, CNDD- Comores Luc Gigord - Conservatoire botanique du Mascarin, Réunion Claude-Anne Gauthier, Muséum National d‘Histoire Naturelle, Paris Jean-Paul Gaudechoux, Commission de l‘Océan Indien Drafted by the Ecosystem Profiling Team: Pierre Carret (CEPF) Harison Rabarison, Nirhy Rabibisoa, Setra Andriamanaitra, -

AP ART HISTORY--Unit 5 Study Guide (Non Western Art Or Art Beyond the European Tradition) Ms
AP ART HISTORY--Unit 5 Study Guide (Non Western Art or Art Beyond the European Tradition) Ms. Kraft BUDDHISM Important Chronology in the Study of Buddhism Gautama Sakyamuni born ca. 567 BCE Buddhism germinates in the Ganges Valley ca. 487-275 BCE Reign of King Ashoka ca. 272-232 BCE First Indian Buddha image ca. 1-200 CE First known Chinese Buddha sculpture 338 CE First known So. China Buddha image 437 CE The Four Noble Truthsi 1. Life means suffering. Life is full of suffering, full of sickness and unhappiness. Although there are passing pleasures, they vanish in time. 2. The origin of suffering is attachment. People suffer for one simple reason: they desire things. It is greed and self-centeredness that bring about suffering. Desire is never satisfied. 3. The cessation of suffering is attainable. It is possible to end suffering if one is aware of his or her own desires and puts and end to them. This awareness will open the door to lasting peace. 4. The path to the cessation of suffering. By changing one’s thinking and behavior, a new awakening can be reached by following the Eightfold Path. Holy Eightfold Pathii The principles are • Right Understanding • Right Intention • Right Speech • Right Action • Right Livelihood • Right Effort • Right Awareness • Right Concentration Following the Eightfold Path will lead to Nirvana or salvation from the cycle of rebirth. Iconography of the Buddhaiii The image of the Buddha is distinguished in various different ways. The Buddha is usually shown in a stylized pose or asana. Also important are the 32 lakshanas or special bodily features or birthmarks. -

AFROTHERIAN CONSERVATION Newsletter of the IUCN/SSC Afrotheria Specialist Group
AFROTHERIAN CONSERVATION Newsletter of the IUCN/SSC Afrotheria Specialist Group Number 10 Edited by PJ Stephenson September 2014 Afrotherian Conservation is published annually by the Speaking of our website, it was over ten years old IUCN Species Survival Commission Afrotheria Specialist and suffering from outdated material and old technology, Group to promote the exchange of news and inform- making it very difficult to maintain. Charles Fox, who ation on the conservation of, and applied research into, does our web maintenance at a hugely discounted cost golden moles, sengis, hyraxes, tenrecs and the aardvark. (many thanks Charles), has reworked the site, especially the design of the home page and conservation page Published by IUCN, Gland, Switzerland. (thanks to Rob Asher for his past efforts with the latter © 2014 International Union for Conservation of Nature material, which is still the basis for the new conservation and Natural Resources page). Because some of the hyrax material was dated, Lee ISSN: 1664-6754 Koren and her colleagues completely updated the hyrax material, and we have now linked our websites. A similar Find out more about the Group on our website at update is being discussed by Tom Lehmann and his http://afrotheria.net/ASG.html and follow us on colleagues for the aardvark link. The sengi web material is Twitter @Tweeting_Tenrec largely unchanged, with the exception of updating various pages to accommodate the description of a new species from Namibia (go to the current topics tab in the Message from the Chair sengi section). Galen Rathbun Although a lot of effort has focused on our Chair, IUCN/SSC Afrotheria Specialist Group group's education goals (logo, website, newsletter), it has not over-shadowed one of the other major functions that There has been a long time gap since our last newsletter our specialist group performs: the periodic update of the was produced in October 2012. -

Major Geographic Qualities of South Asia
South Asia Chapter 8, Part 1: pages 402 - 417 Teacher Notes DEFINING THE REALM I. Major geographic qualities of South Asia - Well defined physiography – bordered by mountains, deserts, oceans - Rivers – The Ganges supports most of population for over 10,000 years - World’s 2nd largest population cluster on 3% land mass - Current birth rate will make it 1st in population in a decade - Low income economies, inadequate nutrition and poor health - British imprint is strong – see borders and culture - Monsoons, this cycle sustains life, to alter it would = disaster - Strong cultural regionalism, invading armies and cultures diversified the realm - Hindu, Buddhists, Islam – strong roots in region - India is biggest power in realm, but have trouble with neighbors - Kashmir – dangerous source of friction between 2 nuclear powers II. Defining the Realm Divided along arbitrary lines drawn by England Division occurred in 1947 and many lives were lost This region includes: Pakistan (East & West), India, Bangladesh (1971), Sri Lanka, Maldives Islands Language – English is lingua franca III. Physiographic Regions of South Asia After Russia left Afghanistan Islamic revivalism entered the region Enormous range of ecologies and environment – Himalayas, desert and tropics 1) Monsoons – annual rains that are critical to life in that part of the world 1 2) Regions: A. Northern Highlands – Himalayas, Bhutan, Afghanistan B. River Lowlands – Indus Valley in Pakistan, Ganges Valley in India, Bangladesh C. Southern Plateaus – throughout much of India, a rich -

Informes Individuales IUCN 2018.Indd
IUCN SSC Afrotheria Specialist Group 2018 Report Galen Rathbun Andrew Taylor Co-Chairs Mission statement of golden moles in species where it is neces- Galen Rathbun (1) The IUCN SSC Afrotheria Specialist Group (ASG) sary (e.g., Amblysomus and Neamblysomus Andrew Taylor (2) facilitates the conservation of hyraxes, aard- species); (3) collect basic data for 3-4 golden varks, elephant-shrews or sengis, golden moles, mole species, including geographic distributions Red List Authority Coordinator tenrecs and their habitats by: (1) providing and natural history data; (4) conduct surveys to determine distribution and abundance of Matthew Child (3) sound scientific advice and guidance to conser- vationists, governments, and other inter- five hyrax species; (5) revise taxonomy of five hyrax species; (6) develop and assess field trials Location/Affiliation ested groups; (2) raising public awareness; for standardised camera trapping methods (1) California Academy of Sciences, and (3) developing research and conservation to determine population estimates for giant California, US programmes. sengis; (7) conduct surveys to assess distribu- (2) The Endangered Wildlife Trust, tion, abundance, threats and taxonomic status Modderfontein, Johannesburg, South Africa Projected impact for the 2017-2020 of the Data Deficient sengi species; (8) build on (3) South African National Biodiversity Institute quadrennium current research to determine the systematics (SANBI), Kirstenbosch National Botanical If the ASG achieved all of its targets, it would be of giant sengis, especially Rhynchocyon Garden, Newlands Cape Town, South Africa able to deliver more accurate, data-driven Red species; (9) survey Aardvark (Orycteropus afer) List assessments for more Afrotherian species populations to determine abundance, distribu- Number of members and, therefore, be in a better position to move tion and trends; (10) conduct taxonomic studies 34 to conservation planning, especially for priority to determine the systematics of aardvarks, with species. -

Chromosomal Evolution in Tenrecs (Microgale and Oryzorictes, Tenrecidae) from the Central Highlands of Madagascar
Chromosome Research (2007) 15:1075–1091 # Springer 2007 DOI: 10.1007/s10577-007-1182-6 Chromosomal evolution in tenrecs (Microgale and Oryzorictes, Tenrecidae) from the Central Highlands of Madagascar C. Gilbert1, S. M. Goodman2,3, V. Soarimalala3,4, L. E. Olson5,P.C.M.O_Brien6, F. F. B. Elder7, F. Yang8, M. A. Ferguson-Smith6 & T. J. Robinson1* 1Evolutionary Genomics Group, Department of Botany and Zoology, University of Stellenbosch, Stellenbosch, South Africa; Tel: +27-21-8083955; Fax: +27-21-8082405; E-mail: [email protected]; 2Department of Zoology, Field Museum of Natural History, Lake Shore Drive, Chicago, IL, USA; 3Vahatra, BP 738, Antananarivo (101), Madagascar; 4De´partement de Biologie Animale, Universite´ d_Antananarivo, BP 906, Antananarivo (101), Madagascar; 5University of Alaska Museum, University of Alaska Fairbanks, Fairbanks, AK, USA; 6Centre for Veterinary Science, University of Cambridge, Cambridge, UK; 7Department of Pathology, Cytogenetics Laboratory, UT Southwestern Medical Center, Dallas, TX, USA; 8The Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, UK *Correspondence Received 13 August 2007. Received in revised form and accepted for publication by Pat Heslop-Harrison 2 October 2007 Key words: Afrotheria, cytogenetics, evolution, speciation, Tenrecidae Abstract Tenrecs (Tenrecidae) are a widely diversified assemblage of small eutherian mammals that occur in Madagascar and Western and Central Africa. With the exception of a few early karyotypic descriptions based on conventional staining, nothing is known about the chromosomal evolution of this family. We present a detailed analysis of G-banded and molecularly defined chromosomes based on fluorescence in situ hybridization (FISH) that allows a comprehensive comparison between the karyotypes of 11 species of two closely related Malagasy genera, Microgale (10 species) and Oryzorictes (one species), of the subfamily Oryzorictinae. -
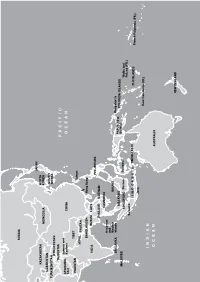
Asia and Oceania Nicole Girard, Irwin Loy, Marusca Perazzi, Jacqui Zalcberg the Country
ARCTIC OCEAN RUSSIA JAPAN KAZAKHSTAN NORTH MONGOLIA KOREA UZBEKISTAN SOUTH TURKMENISTAN KOREA KYRGYZSTAN TAJIKISTAN PACIFIC Jammu and AFGHANIS- Kashmir CHINA TAN OCEAN PAKISTAN TIBET Taiwan NEPAL BHUTAN BANGLADESH Hong Kong INDIA BURMA LAOS PHILIPPINES THAILAND VIETNAM CAMBODIA Andaman and Nicobar BRUNEI SRI LANKA Islands Bougainville MALAYSIA PAPUA NEW SOLOMON ISLANDS MALDIVES GUINEA SINGAPORE Borneo Sulawesi Wallis and Futuna (FR.) Sumatra INDONESIA TIMOR-LESTE FIJI ISLANDS French Polynesia (FR.) Java New Caledonia (FR.) INDIAN OCEAN AUSTRALIA NEW ZEALAND Asia and Oceania Nicole Girard, Irwin Loy, Marusca Perazzi, Jacqui Zalcberg the country. However, this doctrine is opposed by nationalist groups, who interpret it as an attack on ethnic Kazakh identity, language and Central culture. Language policy is part of this debate. The Asia government has a long-term strategy to gradually increase the use of Kazakh language at the expense Matthew Naumann of Russian, the other official language, particularly in public settings. While use of Kazakh is steadily entral Asia was more peaceful in 2011, increasing in the public sector, Russian is still with no repeats of the large-scale widely used by Russians, other ethnic minorities C violence that occurred in Kyrgyzstan and many urban Kazakhs. Ninety-four per cent during the previous year. Nevertheless, minor- of the population speak Russian, while only 64 ity groups in the region continue to face various per cent speak Kazakh. In September, the Chair forms of discrimination. In Kazakhstan, new of the Kazakhstan Association of Teachers at laws have been introduced restricting the rights Russian-language Schools reportedly stated in of religious minorities. Kyrgyzstan has seen a a roundtable discussion that now 56 per cent continuation of harassment of ethnic Uzbeks in of schoolchildren study in Kazakh, 33 per cent the south of the country, and pressure over land in Russian, and the rest in smaller minority owned by minority ethnic groups. -

Albania's 'Sworn Virgins'
THE LINGUISTIC EXPRESSION OF GENDER IDENTITY: ALBANIA’S ‘SWORN VIRGINS’ CARLY DICKERSON A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS GRADUATE PROGRAM IN LINGUISTICS YORK UNIVERSITY TORONTO, ONTARIO August 2015 © Carly Dickerson, 2015 Abstract This paper studies the linguistic tools employed in the construction of masculine identities by burrneshat (‘sworn virgins’) in northern Albania: biological females who have become ‘social men’. Unlike other documented ‘third genders’ (Kulick 1999), burrneshat are not motivated by considerations of personal identity or sexual desire, but rather by the need to fulfill patriarchal roles within a traditional social code that views women as property. Burrneshat are thus seen as honourable and self-sacrificing, are accepted as men in their community, and are treated accordingly, except that they do not marry or engage in sexual relationships. Given these unique circumstances, how do the burrneshat construct and express their identity linguistically, and how do others within the community engage with this identity? Analysis of the choices of grammatical gender in the speech of burrneshat and others in their communities indicates both inter- and intra-speaker variation that is linked to gendered ideologies. ii Table of Contents Abstract ………………………………………………………………………………………….. ii Table of Contents ……………………………………………………………………………….. iii List of Tables …………………………………………………………………………..……… viii List of Figures ……………………………………………………………………………………ix Chapter One – Introduction ……………………………………………………………………... 1 Chapter Two – Albanian People and Language ………………………………………………… 6 2.0 Introduction ………………………………………………………………………………6 2.1 History of Albania ………………………………………………………………………..6 2.1.1 Geographical Location ……………………………………………………………..6 2.1.2 Illyrian Roots ……………………………………………………………………….7 2.1.3 A History of Occupations …………………………………………………………. 8 2.1.4 Northern Albania …………………………………………………………………. -

(Mammalia) from the Eocene of Black Crow, Namibia Martin PICKFORD
Tiny Tenrecomorpha (Mammalia) from the Eocene of Black Crow, Namibia Martin PICKFORD Sorbonne Universités (CR2P, MNHN, CNRS, UPMC - Paris VI) 8, rue Buffon, 75005, Paris, France, (e-mail : [email protected]) Abstract: The 2019 campaign of acid treatment of Eocene freshwater limestone from Black Crow, Namibia, resulted in the recovery of a minuscule mandible of an insectivoran-grade mammal representing a new genus and species of Tenrecomorpha. The specimen is the smallest mammal described from the fossil record from Africa. From the incisor alveoli to the rear end of the angle, the jaw measures a mere 8.6 mm. The jaw is relatively complete, but has lost the incisors, canine and p/2. It shows several characters that link it to the suborder Tenrecomorpha. In some morphological features it recalls Tenrecidae, in others Potamogalidae. The new genus and species throws doubt on the homogeneity of the order Afroinsectiphilia, which in its turn renders doubtful the concept of Afrotheria as currently understood. Key words: Tenrec, Ypresian/Lutetian, Mandible, Namibia To cite this paper: Pickford, M. 2019. Tiny Tenrecomorpha (Mammalia) from the Eocene of Black Crow, Namibia. Communications of the Geological Survey of Namibia, 21, 15-25. Introduction This paper is devoted to the description and Each year the Namibia Palaeontology interpretation of a minuscule mammalian Expedition has visited the locality to search for mandible from the middle Eocene limestones at blocks of limestone showing the presence of Black Crow, Namibia. The freshwater bones and teeth on their surfaces and these have limestone at Black Crow in the Sperrgebiet, been developed in the laboratory to extract the Namibia, first yielded vertebrate fossils a fossils. -

Morphological Diversity in Tenrecs (Afrosoricida, Tenrecidae)
Morphological diversity in tenrecs (Afrosoricida, Tenrecidae): comparing tenrec skull diversity to their closest relatives Sive Finlay and Natalie Cooper School of Natural Sciences, Trinity College Dublin, Dublin, Ireland Trinity Centre for Biodiversity Research, Trinity College Dublin, Dublin, Ireland ABSTRACT It is important to quantify patterns of morphological diversity to enhance our un- derstanding of variation in ecological and evolutionary traits. Here, we present a quantitative analysis of morphological diversity in a family of small mammals, the tenrecs (Afrosoricida, Tenrecidae). Tenrecs are often cited as an example of an ex- ceptionally morphologically diverse group. However, this assumption has not been tested quantitatively. We use geometric morphometric analyses of skull shape to test whether tenrecs are more morphologically diverse than their closest relatives, the golden moles (Afrosoricida, Chrysochloridae). Tenrecs occupy a wider range of ecological niches than golden moles so we predict that they will be more morpho- logically diverse. Contrary to our expectations, we find that tenrec skulls are only more morphologically diverse than golden moles when measured in lateral view. Furthermore, similarities among the species-rich Microgale tenrec genus appear to mask higher morphological diversity in the rest of the family. These results reveal new insights into the morphological diversity of tenrecs and highlight the impor- tance of using quantitative methods to test qualitative assumptions about patterns of morphological diversity. Submitted 29 January 2015 Subjects Evolutionary Studies, Zoology Accepted 13 April 2015 Keywords Golden moles, Geometric morphometrics, Disparity, Morphology Published 30 April 2015 Corresponding author Natalie Cooper, [email protected] INTRODUCTION Academic editor Analysing patterns of morphological diversity (the variation in physical form Foote, Laura Wilson 1997) has important implications for our understanding of ecological and evolutionary Additional Information and traits. -

Myzopodidae: Chiroptera) from Western Madagascar
ARTICLE IN PRESS www.elsevier.de/mambio Original investigation The description of a new species of Myzopoda (Myzopodidae: Chiroptera) from western Madagascar By S.M. Goodman, F. Rakotondraparany and A. Kofoky Field Museum of Natural History, Chicago, USA and WWF, Antananarivo, De´partement de Biologie Animale, Universite´ d’Antananarivo, Antananarivo, Madagasikara Voakajy, Antananarivo, Madagascar Receipt of Ms. 6.2.2006 Acceptance of Ms. 2.8.2006 Abstract A new species of Myzopoda (Myzopodidae), an endemic family to Madagascar that was previously considered to be monospecific, is described. This new species, M. schliemanni, occurs in the dry western forests of the island and is notably different in pelage coloration, external measurements and cranial characters from M. aurita, the previously described species, from the humid eastern forests. Aspects of the biogeography of Myzopoda and its apparent close association with the plant Ravenala madagascariensis (Family Strelitziaceae) are discussed in light of possible speciation mechanisms that gave rise to eastern and western species. r 2006 Deutsche Gesellschaft fu¨r Sa¨ugetierkunde. Published by Elsevier GmbH. All rights reserved. Key words: Myzopoda, Madagascar, new species, biogeography Introduction Recent research on the mammal fauna of speciation molecular studies have been very Madagascar has and continues to reveal informative to resolve questions of species remarkable discoveries. A considerable num- limits (e.g., Olson et al. 2004; Yoder et al. ber of new small mammal and primate 2005). The bat fauna of the island is no species have been described in recent years exception – until a decade ago these animals (Goodman et al. 2003), and numerous remained largely under studied and ongoing other mammals, known to taxonomists, surveys and taxonomic work have revealed await formal description. -

Endemic Small Mammals Captured Outside of Natural Habitats in the Moramanga District, Central Eastern Madagascar
Terrestrial “forest-dwelling” endemic small mammals captured outside of natural habitats in the Moramanga District, central eastern Madagascar Toky M. Randriamoria1,2, Voahangy Soarimalala2 petits mammifères, par le biais des trous-pièges et & Steven M. Goodman2, 3 des pièges standards, menées dans cinq villages du 1Département de Biologie Animale, Faculté des District de Moramanga, dans la partie Est-centrale Sciences, Université d’Antananarivo, BP 906, de Madagascar, en 2013 (saison sèche) et en 2014 Antananarivo 101, Madagascar (saison humide) ont permis d’attraper pour la première E-mail: [email protected] fois deux espèces d’Afrosoricida endémiques, 2 Association Vahatra, BP 3972, Antananarivo 101, Microgale majori et M. thomasi, dans des habitats Madagascar anthropogéniques en dehors des forêts naturelles. E-mail: [email protected] La première espèce a été répertoriée dans deux 3Field Museum of Natural History, 1400 South Lake sites (Ambalafary et Antsahatsaka) à travers deux Shore Drive, Chicago, Illinois 60605, USA types d’habitats principaux : la forêt d’Eucalyptus et E-mail: [email protected] le savoka. Le terme savoka désigne des formations végétales secondaires souvent peu pénétrables et correspond à la période de jachère des cultures sur Abstract brûlis. Ces habitats de capture sont situés près de The vast majority of Malagasy rodent (Subfamily 770 m jusqu’à plus de 3 km d’une forêt naturelle. Nesomyinae) and tenrec (Subfamily Oryzorictinae) Concernant M. thomasi, un spécimen a été capturé species living in the eastern humid forest are thought dans un savoka situé à près d’une centaine de to be strictly forest-dwelling. Small mammals surveys mètres d’une forêt naturelle, dans le site de Besakay.