Organic Chemistry 2 – Spring 2021 Week 10 Resource Hello Everyone!
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

De Novo Biosynthesis of Terminal Alkyne-Labeled Natural Products
De novo biosynthesis of terminal alkyne-labeled natural products Xuejun Zhu1,2, Joyce Liu2,3, Wenjun Zhang1,2,4* 1Department of Chemical and Biomolecular Engineering, 2Energy Biosciences Institute, 3Department of Bioengineering, University of California, Berkeley, CA 94720, USA. 4Physical Biosciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA. *e-mail:[email protected] 1 Abstract: The terminal alkyne is a functionality widely used in organic synthesis, pharmaceutical science, material science, and bioorthogonal chemistry. This functionality is also found in acetylenic natural products, but the underlying biosynthetic pathways for its formation are not well understood. Here we report the characterization of the first carrier protein- dependent terminal alkyne biosynthetic machinery in microbes. We further demonstrate that this enzymatic machinery can be exploited for the in situ generation and incorporation of terminal alkynes into two natural product scaffolds in E. coli. These results highlight the prospect for tagging major classes of natural products, including polyketides and polyketide/non-ribosomal peptide hybrids, using biosynthetic pathway engineering. 2 Natural products are important small molecules widely used as drugs, pesticides, herbicides, and biological probes. Tagging natural products with a unique chemical handle enables the visualization, enrichment, quantification, and mode of action study of natural products through bioorthogonal chemistry1-4. One prevalent bioorthogonal reaction is -

Part I: Carbonyl-Olefin Metathesis of Norbornene
Part I: Carbonyl-Olefin Metathesis of Norbornene Part II: Cyclopropenimine-Catalyzed Asymmetric Michael Reactions Zara Maxine Seibel Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2016 1 © 2016 Zara Maxine Seibel All Rights Reserved 2 ABSTRACT Part I: Carbonyl-Olefin Metathesis of Norbornene Part II: Cyclopropenimine-Catalyzed Asymmetric Michael Reactions Zara Maxine Seibel This thesis details progress towards the development of an organocatalytic carbonyl- olefin metathesis of norbornene. This transformation has not previously been done catalytically and has not been done in practical manner with stepwise or stoichiometric processes. Building on the previous work of the Lambert lab on the metathesis of cyclopropene and an aldehyde using a hydrazine catalyst, this work discusses efforts to expand to the less stained norbornene. Computational and experimental studies on the catalytic cycle are discussed, including detailed experimental work on how various factors affect the difficult cycloreversion step. The second portion of this thesis details the use of chiral cyclopropenimine bases as catalysts for asymmetric Michael reactions. The Lambert lab has previously developed chiral cyclopropenimine bases for glycine imine nucleophiles. The scope of these catalysts was expanded to include glycine imine derivatives in which the nitrogen atom was replaced with a carbon atom, and to include imines derived from other amino acids. i Table of Contents List of Abbreviations…………………………………………………………………………..iv Part I: Carbonyl-Olefin Metathesis…………………………………………………………… 1 Chapter 1 – Metathesis Reactions of Double Bonds………………………………………….. 1 Introduction………………………………………………………………………………. 1 Olefin Metathesis………………………………………………………………………… 2 Wittig Reaction…………………………………………………………………………... 6 Tebbe Olefination………………………………………………………………………... 9 Carbonyl-Olefin Metathesis……………………………………………………………. -

Phospholipid:Diacylglycerol Acyltransferase: an Enzyme That Catalyzes the Acyl-Coa-Independent Formation of Triacylglycerol in Yeast and Plants
Phospholipid:diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants Anders Dahlqvist*†‡, Ulf Ståhl†§, Marit Lenman*, Antoni Banas*, Michael Lee*, Line Sandager¶, Hans Ronne§, and Sten Stymne¶ *Scandinavian Biotechnology Research (ScanBi) AB, Herman Ehles Va¨g 2 S-26831 Svaloˆv, Sweden; ¶Department of Plant Breeding Research, Swedish University of Agricultural Sciences, Herman Ehles va¨g 2–4, S-268 31 Svalo¨v, Sweden; and §Department of Plant Biology, Uppsala Genetic Center, Swedish University of Agricultural Sciences, Box 7080, S-750 07 Uppsala, Sweden Edited by Christopher R. Somerville, Carnegie Institution of Washington, Stanford, CA, and approved March 31, 2000 (received for review February 15, 2000) Triacylglycerol (TAG) is known to be synthesized in a reaction that acid) and epoxidated fatty acid (vernolic acid) in TAG in castor uses acyl-CoA as acyl donor and diacylglycerol (DAG) as acceptor, bean (Ricinus communis) and the hawk’s-beard Crepis palaestina, and which is catalyzed by the enzyme acyl-CoA:diacylglycerol respectively. Furthermore, a similar enzyme is shown to be acyltransferase. We have found that some plants and yeast also present in the yeast Saccharomyces cerevisiae, and the gene have an acyl-CoA-independent mechanism for TAG synthesis, encoding this enzyme, YNR008w, is identified. which uses phospholipids as acyl donors and DAG as acceptor. This reaction is catalyzed by an enzyme that we call phospholipid:dia- Materials and Methods cylglycerol acyltransferase, or PDAT. PDAT was characterized in Yeast Strains and Plasmids. The wild-type yeast strains used were microsomal preparations from three different oil seeds: sunflower, either FY1679 (MAT␣ his3-⌬200 leu2-⌬1 trp1-⌬6 ura3-52) (9) or castor bean, and Crepis palaestina. -

Amide Activation: an Emerging Tool for Chemoselective Synthesis
Featuring work from the research group of Professor As featured in: Nuno Maulide, University of Vienna, Vienna, Austria Amide activation: an emerging tool for chemoselective synthesis Let them stand out of the crowd – Amide activation enables the chemoselective modification of a large variety of molecules while leaving many other functional groups untouched, making it attractive for the synthesis of sophisticated targets. This issue features a review on this emerging field and its application in total synthesis. See Nuno Maulide et al., Chem. Soc. Rev., 2018, 47, 7899. rsc.li/chem-soc-rev Registered charity number: 207890 Chem Soc Rev View Article Online REVIEW ARTICLE View Journal | View Issue Amide activation: an emerging tool for chemoselective synthesis Cite this: Chem. Soc. Rev., 2018, 47,7899 Daniel Kaiser, Adriano Bauer, Miran Lemmerer and Nuno Maulide * It is textbook knowledge that carboxamides benefit from increased stabilisation of the electrophilic carbonyl carbon when compared to other carbonyl and carboxyl derivatives. This results in a considerably reduced reactivity towards nucleophiles. Accordingly, a perception has been developed of amides as significantly less useful functional handles than their ester and acid chloride counterparts. Received 27th April 2018 However, a significant body of research on the selective activation of amides to achieve powerful DOI: 10.1039/c8cs00335a transformations under mild conditions has emerged over the past decades. This review article aims at placing electrophilic amide activation in both a historical context and in that of natural product rsc.li/chem-soc-rev synthesis, highlighting the synthetic applications and the potential of this approach. Creative Commons Attribution 3.0 Unported Licence. -

2 Reactions Observed with Alkanes Do Not Occur with Aromatic Compounds 2 (SN2 Reactions Never Occur on Sp Hybridized Carbons!)
Reactions of Aromatic Compounds Aromatic compounds are stabilized by this “aromatic stabilization” energy Due to this stabilization, normal SN2 reactions observed with alkanes do not occur with aromatic compounds 2 (SN2 reactions never occur on sp hybridized carbons!) In addition, the double bonds of the aromatic group do not behave similar to alkene reactions Aromatic Substitution While aromatic compounds do not react through addition reactions seen earlier Br Br Br2 Br2 FeBr3 Br With an appropriate catalyst, benzene will react with bromine The product is a substitution, not an addition (the bromine has substituted for a hydrogen) The product is still aromatic Electrophilic Aromatic Substitution Aromatic compounds react through a unique substitution type reaction Initially an electrophile reacts with the aromatic compound to generate an arenium ion (also called sigma complex) The arenium ion has lost aromatic stabilization (one of the carbons of the ring no longer has a conjugated p orbital) Electrophilic Aromatic Substitution In a second step, the arenium ion loses a proton to regenerate the aromatic stabilization The product is thus a substitution (the electrophile has substituted for a hydrogen) and is called an Electrophilic Aromatic Substitution Energy Profile Transition states Transition states Intermediate Potential E energy H Starting material Products E Reaction Coordinate The rate-limiting step is therefore the formation of the arenium ion The properties of this arenium ion therefore control electrophilic aromatic substitutions (just like any reaction consider the stability of the intermediate formed in the rate limiting step) 1) The rate will be faster for anything that stabilizes the arenium ion 2) The regiochemistry will be controlled by the stability of the arenium ion The properties of the arenium ion will predict the outcome of electrophilic aromatic substitution chemistry Bromination To brominate an aromatic ring need to generate an electrophilic source of bromine In practice typically add a Lewis acid (e.g. -

Organic Chemistry/Fourth Edition: E-Text
CHAPTER 17 ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION TO THE CARBONYL GROUP O X ldehydes and ketones contain an acyl group RC± bonded either to hydrogen or Ato another carbon. O O O X X X HCH RCH RCRЈ Formaldehyde Aldehyde Ketone Although the present chapter includes the usual collection of topics designed to acquaint us with a particular class of compounds, its central theme is a fundamental reaction type, nucleophilic addition to carbonyl groups. The principles of nucleophilic addition to alde- hydes and ketones developed here will be seen to have broad applicability in later chap- ters when transformations of various derivatives of carboxylic acids are discussed. 17.1 NOMENCLATURE O X The longest continuous chain that contains the ±CH group provides the base name for aldehydes. The -e ending of the corresponding alkane name is replaced by -al, and sub- stituents are specified in the usual way. It is not necessary to specify the location of O X the ±CH group in the name, since the chain must be numbered by starting with this group as C-1. The suffix -dial is added to the appropriate alkane name when the com- pound contains two aldehyde functions.* * The -e ending of an alkane name is dropped before a suffix beginning with a vowel (-al) and retained be- fore one beginning with a consonant (-dial). 654 Back Forward Main Menu TOC Study Guide TOC Student OLC MHHE Website 17.1 Nomenclature 655 CH3 O O O O CH3CCH2CH2CH CH2 CHCH2CH2CH2CH HCCHCH CH3 4,4-Dimethylpentanal 5-Hexenal 2-Phenylpropanedial When a formyl group (±CHœO) is attached to a ring, the ring name is followed by the suffix -carbaldehyde. -

Detection of Phenethylamine, Amphetamine, and Tryptamine Imine By-Products from an Acetone Extraction
Detection of Phenethylamine, Amphetamine, and Tryptamine Imine By-Products from an Acetone Extraction Mary A. Yohannan* and Arthur Berrier U.S. Department of Justice Drug Enforcement Administration Special Testing and Research Laboratory 22624 Dulles Summit Court Dulles, VA 20166 [email: mary.a.yohannan -at- usdoj.gov] ABSTRACT: The formation of imine by-products from phenethylamines, amphetamines, and tryptamines upon an acetone extraction is presented. These imine by-products were characterized using GC/MSD and exhibited preferential cleavage at the α-carbon of the alkyl chain. Further characterization of the imine by-products of phenethylamine and tryptamine was done using IR and NMR. KEYWORDS: phenethylamine, tryptamine, imine, acetone, schiff base, drug chemistry, forensic chemistry In most forensic laboratories, the solvents used to extract at the α-carbon on the alkyl chain. In addition to GC/MS, the drugs are chosen based upon their solubility properties and their imines formed from phenethylamine base and tryptamine base ability to not interact with the drug. In fact, there are very few were characterized by Fourier transform-infrared spectroscopy publications where a solvent used to extract a drug reacts with (FTIR) and nuclear magnetic resonance (NMR) spectroscopy. the drug and forms by-products [1-3]. This laboratory recently discovered that an additional Experimental component was formed when acetone was used to extract a Solvents, Chemicals, and Materials sample containing a known tryptamine. Analysis by gas Acetone was ACS/HPLC grade from Burdick and Jackson chromatography/mass spectroscopy (GC/MS) of the acetone Laboratories (Muskegon, MI). Phenethylamine base and extract yielded an extra peak in the total ion chromatogram that tryptamine base were obtained from Sigma-Aldrich Chemicals was approximately half the abundance of the known tryptamine (Milwaukee, WI). -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

Using Dynamic Combinatorial Chemistry to Construct Novel Thioester and Disulfide Lipids
Using dynamic combinatorial chemistry to construct novel thioester and disulfide lipids A dissertation submitted to the University of Manchester for the degree of MSc by Research Chemistry In the Faculty of Science and Engineering 2017 Jack A. Yung School of Chemistry Table of Contents List of figures, tables and equations 5 Symbols and abbreviations 10 Abstract 12 Declaration 13 Copyright statement 13 Acknowledgements 14 The author 14 Chapter 1. Introduction 15 1.1 The cell membrane 16 1.1.1 Function and composition 16 1.2 Membrane lipids 16 1.2.1 Lipid classification 16 1.2.2 Amphiphiles 17 1.2.3 Glycolipids 17 1.2.4 Sterols 18 1.2.5 Phospholipids 19 1.3 Lipid vesicles 20 1.3.1 Supramolecular self-assembly 20 1.3.2 Interaction free energies 21 1.3.3 Framework for the theory of self-assembly 22 1.3.4 Micelles 23 1.3.5 Lipid bilayers 24 1.3.6 Vesicles 25 1.3.7 Phase-transition temperature 27 1.4 Amphiphilic building blocks 27 1.5 Thioesters 29 1.5.1 Thioester reactivity 29 1.5.2 Trans-thioesterification 30 1.5.3 Thioester exchange reactions in DCC 31 1.6 Disulfides 32 2 1.6.1 Disulfide reactivity 32 1.6.2 Thiol-disulfide interchange reactions 32 1.6.3 Disulfide exchange reactions in DCC 33 1.7 Pre-biotic lipids 34 1.7.1 Sources of pre-biotic organic compounds 34 1.7.2 The first pre-biotic membrane structure 36 1.7.3 The role of sulfur in pre-biotic chemistry 36 1.8 Artificially designed vesicles 37 1.8.1 Applications of artificially designed vesicles 37 1.8.2 Zeta-potential 37 1.8.3 Vesicle design 38 1.9 Targets 39 1.9.1 Aims 39 Chapter 2. -

The Carcinogenicity of the O-Methoxy Derivatives of N-2-Fluorenylacetamide and of Related Compounds in the Rat
[CANCER RESEARCH 28, 234-244, February 1968] The Carcinogenicity of the o-Methoxy Derivatives of N-2-Fluorenylacetamide and of Related Compounds in the Rat H. R. Gutmann, S. B. Galitski, and W. A. Foley Laboratory ]or Cancer Research, Veterans Administration Hospital, and Department o] Biochemistry, University o] Minnesota, Minneapolis, Minnesota 55417 SUMMARY acetamide by the sequential reactions of deacetylation and oxidation. In order to test the idea that the lack of carcinogenicity of the o-amidofluorenols, N- (1-hydroxy-2-fluorenyl) acetamide and INTRODUCTION N-(3-hydroxy-2-fluorenyl)acetamide, is due to the hydrophilic phenolic hydroxyl group, the methylated derivatives, N-(1- Several model studies from this laboratory have shown that methoxy-2-fluorenyl)acetamide and N-(3-methoxy-2-fluorenyl) the o-quinone imines, 2-imino-l,2-fluorenoquinone and 2-imino- acetamide as well as the hydrochlorides of 1-methoxy-2-fluo- 2,3-fluorenoquinone, which are derived from the carcinogen renamine and 3-methoxy-2-fiuorenamine, were prepared, and N-2-fluorenylacetamide by the sequential enzymatic reactions their carcinogenicity was evaluated in the rat. N-(1-Methoxy- of hydroxylation, deacetylation, and oxidation (6, 10, 12, 27, 2-fluorenyl)acetamide and 1-methoxy-2-fluorenamine hydro- 34, 35), form stable adducts with a variety of proteins (17, 18). chloride, when administered orally to male rats for 5 months, However, the relevance of the binding of these o-quinone imines gave a tumor incidence of 27 and 50%, respectively. Approxi- to chemical carcinogenesis has remained obscure largely be- mately one-half of the lesions produced by either com- cause the o-amidofluorenols, 1-OH-AAF 2 and 3-OH-AAF pound were adenocarcinomas of the small intestine. -
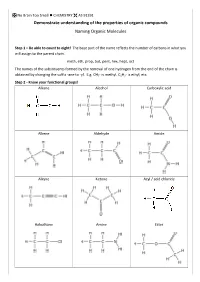
IUPAC Naming
No Brain Too Small CHEMISTRY AS 91391 Demonstrate understanding of the properties of organic compounds Naming Organic Molecules Step 1 – Be able to count to eight! The base part of the name reflects the number of carbons in what you will assign to the parent chain. meth, eth, prop, but, pent, hex, hept, oct The names of the substituents formed by the removal of one hydrogen from the end of the chain is obtained by changing the suffix -ane to -yl. E.g. CH3- is methyl, C2H5- is ethyl, etc. Step 2 - Know your functional groups! Alkane Alcohol Carboxylic acid Alkene Aldehyde Amide Alkyne Ketone Acyl / acid chloride Haloalkane Amine Ester No Brain Too Small CHEMISTRY AS 91391 This is NOT an exhaustive list of rules but a guide for L3 NCEA. Identify the principle functional group in the structure. If there is only ONE functional group then this is the principle functional group e.g. CH3CH2CH2COOH will have the suffix -oic acid as the carboxylic acid is the principle functional group. In cases where compounds have more than one functional group, then the principle functional group is decided by a priority order. carboxylic acids > acid derivatives* > aldehydes > ketones > alcohols > amines *esters, acyl/acid chlorides and amides 3-hydroxybutanoic acid 4-aminopentanoic acid as the carboxylic acid functional group takes priority Fluoro-, chloro-, bromo-, iodo- and alkyl groups have “no priority”. Their numbering is governed by the lowest sum rule. 2,3-dichloro-4-methylhexane and NOT 4,5-dichloro-2-methylhexane (as 2+3+4 = 9 whereas 2+4+5 = 11) No Brain Too Small CHEMISTRY AS 91391 Rules 1. -

A User's Guide to the Thiol-Thioester Exchange in Organic Media: Scope, Limitations, and Applications in Material Science
Polymer Chemistry A User's Guide to the Thiol-Thioester Exchange in Organic Media: Scope, Limitations, and Applications in Material Science Journal: Polymer Chemistry Manuscript ID PY-ART-07-2018-001031.R1 Article Type: Paper Date Submitted by the Author: 06-Aug-2018 Complete List of Authors: Worrell, Brady; University of Colorado Boulder, Chemical and Biological Engineering Mavila, Sudheendran; University of Colorado, Department of Chemical and Biological Engineering Wang, Chen; University of Colorado Boulder, Department of Chemical and Biological Engineering Kontour, Taylor; University of Colorado Boulder, Chemical and Biological Engineering Lim, Chern-Hooi; University of Colorado Boulder, Department of Chemical and Biological Engineering McBride, Matthew; University of Colorado Boulder, Department of Chemical and Biological Engineering Musgrave, Charles; University of Colorado, Department of Chemical and Biological Engineering Shoemaker, Richard; University of Colorado, Department of Chemistry and Biochemistry Bowman, Christopher; University of Colorado, Department of Chemical and Biological Engineering Page 1 of 14 PleasePolymer do not Chemistryadjust margins Polymer Chemistry ARTICLE A User’s Guide to the Thiol-Thioester Exchange in Organic Media: Scope, Limitations, and Applications in Material Science P8u8jhReceived 00th January a a a a a 20xx, Brady T. Worrell, Sudheenran Mavila, Chen Wang, Taylor M. Kontour, Chern-Hooi Lim, Accepted 00th January 20xx Matthew K. McBride,a Charles B. Musgrave,a Richard Shoemaker,a Christopher N. Bowmana,b,c,d* DOI: 10.1039/x0xx00000x The exchange of thiolates and thiols has long been held as a nearly ideal reaction in dynamic covalent chemistry. The www.rsc.org/ ability for the reaction to proceed smoothly in neutral aqueous media has propelled its widespread use in biochemistry, however, far fewer applications and studies have been directed towards its use in material science which primarily is performed in organic media.