Larval Development of Shallow Water Barnacles of the Carolinas (Cirripedia: Thoracica) with Keys to Naupliar Stages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Snps) in the Northeast Pacific Intertidal Gooseneck Barnacle, Pollicipes Polymerus
University of Alberta New insights about barnacle reproduction: Spermcast mating, aerial copulation and population genetic consequences by Marjan Barazandeh A thesis submitted to the Faculty of Graduate Studies and Research in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Systematics and Evolution Department of Biological Sciences ©Marjan Barazandeh Spring 2014 Edmonton, Alberta Permission is hereby granted to the University of Alberta Libraries to reproduce single copies of this thesis and to lend or sell such copies for private, scholarly or scientific research purposes only. Where the thesis is converted to, or otherwise made available in digital form, the University of Alberta will advise potential users of the thesis of these terms. The author reserves all other publication and other rights in association with the copyright in the thesis and, except as herein before provided, neither the thesis nor any substantial portion thereof may be printed or otherwise reproduced in any material form whatsoever without the author's prior written permission. Abstract Barnacles are mostly hermaphroditic and they are believed to mate via copulation or, in a few species, by self-fertilization. However, isolated individuals of two species that are thought not to self-fertilize, Pollicipes polymerus and Balanus glandula, nonetheless carried fertilized embryo-masses. These observations raise the possibility that individuals may have been fertilized by waterborne sperm, a possibility that has never been seriously considered in barnacles. Using molecular tools (Single Nucleotide Polymorphisms; SNP), I examined spermcast mating in P. polymerus and B. glandula as well as Chthamalus dalli (which is reported to self-fertilize) in Barkley Sound, British Columbia, Canada. -

(Cirripedia : Thoracica) Over the Body of a Sea Snake, Laticauda Title Semifasciata (Reinwardt), from the Kii Peninsula, Southwestern Japan
Distribution of Two Species of Conchoderma (Cirripedia : Thoracica) over the Body of a Sea Snake, Laticauda Title semifasciata (Reinwardt), from the Kii Peninsula, Southwestern Japan Yamato, Shigeyuki; Yusa, Yoichi; Tanase, Hidetomo; Tanase, Author(s) Hidetomo PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1996), 37(3-6): 337-343 Issue Date 1996-12-25 URL http://hdl.handle.net/2433/176259 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Pub!. Seto Mar. Bioi. Lab., 37(3/6): 337-343, 1996 337 Distribution of Two Species of Conchoderma (Cirripedia: Thoracica) over the Body of a Sea Snake, Laticauda semifasciata (Reinwardt), from the Kii Peninsula, Southwestern Japan SHIGEYUKI YAMATO, YOICHI YUSA and HIDETOMO TANASE Seto Marine Biological Laboratory, Kyoto University, Shirahama, Wakayama 649-22, Japan Abstract Two species of Conchoderma were found on a sea snake, Laticauda semifas ciata (Reinwardt), collected on the west coast of the Kii Peninsula. A total of 223 individuals of C. virgatum and 6 of C. hunteri in 19 clumps were attached to the snake's body. The barnacles ranged in size from 1.4 mm (cypris larvae) to 18.2 mm in capitulum length in C. virgatum, and from 10.7 to 14.4 mm in C. hunteri. The size of the smallest gravid individuals in both species was between 10 and 11 mm. The distribution of C. virgatum on the snake was non-random both longitudinally and dorso-ventrally, with more barnacles in the posterior region and on the ventral side of the snake, respectively. The proportion of gravid individuals increased towards the tail. -

Evolutionary History of Inversions in the Direction of Architecture-Driven
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.09.085712; this version posted May 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Evolutionary history of inversions in the direction of architecture- driven mutational pressures in crustacean mitochondrial genomes Dong Zhang1,2, Hong Zou1, Jin Zhang3, Gui-Tang Wang1,2*, Ivan Jakovlić3* 1 Key Laboratory of Aquaculture Disease Control, Ministry of Agriculture, and State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China. 2 University of Chinese Academy of Sciences, Beijing 100049, China 3 Bio-Transduction Lab, Wuhan 430075, China * Corresponding authors Short title: Evolutionary history of ORI events in crustaceans Abbreviations: CR: control region, RO: replication of origin, ROI: inversion of the replication of origin, D-I skew: double-inverted skew, LBA: long-branch attraction bioRxiv preprint doi: https://doi.org/10.1101/2020.05.09.085712; this version posted May 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Abstract Inversions of the origin of replication (ORI) of mitochondrial genomes produce asymmetrical mutational pressures that can cause artefactual clustering in phylogenetic analyses. It is therefore an absolute prerequisite for all molecular evolution studies that use mitochondrial data to account for ORI events in the evolutionary history of their dataset. -
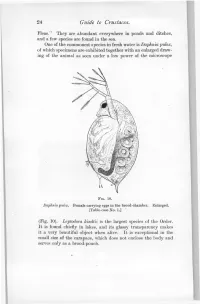
24 Guide to Crustacea
24 Guide to Crustacea. Fleas." They are abundant everywhere in ponds and ditches, and a few species are found in the sea. One of the commonest species in fresh water is Daphnia pulex, of which specimens are exhibited together with an enlarged draw- ing of the animal as seen under a low power of the microscope FIG. 10. Daphnia pulex. Female carrying eggs in the brood-chamber. Enlarged. [Table-case No. 1.] (Fig. 10). Leptodora kindtii is the largest species of the Order. It is found chiefly in lakes, and its glassy transparency makes it a very beautiful object when alive. It is exceptional in the small size of the carapace, which does not enclose the body and serves only as a brood-pouch. Ostracoda. 25 Sub-class II.—OSTRACODA. (Table-ease No. 1.) The number of somites, as indicated by the appendages, is smaller than in any other Crustacea, there being, at most, only two pairs of trunk-limbs behind those belonging to the head- region. The carapace forms a bivalved shell completely en- closing the body and limbs. There is a large, and often leg-like, palp on the mandible. The antennules and antennae are used for creeping or swimming. The Ostracoda (Fig. 10) are for the most part extremely minute animals, and only one or two of the larger species can be exhibited. They occur abundantly in fresh water and in FIG. 11. Shells of Ostracoda, seen from the side. A. Philomedes brenda (Myodocopa) ; B. Cypris fuscata (Podocopa); ('. Cythereis ornata (Podocopa): all much enlarged, n., Notch characteristic of the Myodocopa; e., the median eye ; a., mark of attachment of the muscle connecting the two valves of the shell. -

Larval Development of Shallow Water Barnacles of the Carolinas (Cirripedia
421 NOAA Technical Report Circular 421 OF <•*>" *o, Larval Development of / Shallow Water Barnacles of the Carolinas (Cirripedia: Sr V/ *TES O* Thoracica) With Keys to Naupliar Stages William H. Lang February 1979 U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service NOAA TECHNICAL REPORTS National Marine Fisheries Service, Circulars The major responsibilities of the National Marine Fisheries Service (NMFS) are to monitor and assess the abundance and geographic distribution of fishery resources, to understand and predict fluctuations in the quantity and distribution of these resources, and to establish levels for optimum use of the resources. NMFS is also charged with the development and implementation of policies for managing national fishing grounds, development and enforcement of domestic fisheries regulations, surveillance of foreign fishing off United States coastal waters, and the development and enforcement of international fishery agreements and policies. NMFS also assists the fishing industry through marketing service and economic analysis programs, and mortgage insurance and vessel construction subsidies. It collects, analyzes, and publishes statistics on various phases of the industry. The NOAA Technical Report NMFS Circular series continues a series that has been in existence since 1941. The Circulars are technical publications of general interest intended to aid conservation and management. Publications that review in considerable detail and at a high technical level certain broad areas of research appear in this series. Technical papers originating in economics studies and from management in- vestigations appear in the Circular series. NOAA Technical Report NMFS Circulars are available free in limited numbers to governmental agencies, both Federal and State. -

Balanus Glandula Class: Multicrustacea, Hexanauplia, Thecostraca, Cirripedia
Phylum: Arthropoda, Crustacea Balanus glandula Class: Multicrustacea, Hexanauplia, Thecostraca, Cirripedia Order: Thoracica, Sessilia, Balanomorpha Acorn barnacle Family: Balanoidea, Balanidae, Balaninae Description (the plate overlapping plate edges) and radii Size: Up to 3 cm in diameter, but usually (the plate edge marked off from the parietes less than 1.5 cm (Ricketts and Calvin 1971; by a definite change in direction of growth Kozloff 1993). lines) (Fig. 3b) (Newman 2007). The plates Color: Shell usually white, often irregular themselves include the carina, the carinola- and color varies with state of erosion. Cirri teral plates and the compound rostrum (Fig. are black and white (see Plate 11, Kozloff 3). 1993). Opercular Valves: Valves consist of General Morphology: Members of the Cirri- two pairs of movable plates inside the wall, pedia, or barnacles, can be recognized by which close the aperture: the tergum and the their feathery thoracic limbs (called cirri) that scutum (Figs. 3a, 4, 5). are used for feeding. There are six pairs of Scuta: The scuta have pits on cirri in B. glandula (Fig. 1). Sessile barna- either side of a short adductor ridge (Fig. 5), cles are surrounded by a shell that is com- fine growth ridges, and a prominent articular posed of a flat basis attached to the sub- ridge. stratum, a wall formed by several articulated Terga: The terga are the upper, plates (six in Balanus species, Fig. 3) and smaller plate pair and each tergum has a movable opercular valves including terga short spur at its base (Fig. 4), deep crests for and scuta (Newman 2007) (Figs. -

Hull Fouling Is a Risk Factor for Intercontinental Species Exchange in Aquatic Ecosystems
Aquatic Invasions (2007) Volume 2, Issue 2: 121-131 Open Access doi: http://dx.doi.org/10.3391/ai.2007.2.2.7 © 2007 The Author(s). Journal compilation © 2007 REABIC Research Article Hull fouling is a risk factor for intercontinental species exchange in aquatic ecosystems John M. Drake1,2* and David M. Lodge1,2 1Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46556 USA 2Environmental National Center for Ecological Analysis and Synthesis, 735 State Street, Suite 300, Santa Barbara, CA 93101 USA *Corresponding author Current address: Institute of Ecology, University of Georgia, Athens, GA 30602 USA E-mail: [email protected] (JMD) Received: 13 March 2007 / Accepted: 25 May 2007 Abstract Anthropogenic biological invasions are a leading threat to aquatic biodiversity in marine, estuarine, and freshwater ecosystems worldwide. Ballast water discharged from transoceanic ships is commonly believed to be the dominant pathway for species introduction and is therefore increasingly subject to domestic and international regulation. However, compared to species introductions from ballast, translocation by biofouling of ships’ exposed surfaces has been poorly quantified. We report translocation of species by a transoceanic bulk carrier intercepted in the North American Great Lakes in fall 2001. We collected 944 individuals of at least 74 distinct freshwater and marine taxa. Eight of 29 taxa identified to species have never been observed in the Great Lakes. Employing five different statistical techniques, we estimated that the biofouling community of this ship comprised from 100 to 200 species. These findings adjust upward by an order of magnitude the number of species collected from a single ship. -

Marine Information Network Information on the Species and Habitats Around the Coasts and Sea of the British Isles
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Montagu's stellate barnacle (Chthamalus montagui) MarLIN – Marine Life Information Network Biology and Sensitivity Key Information Review Karen Riley 2002-01-28 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1322]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: Riley, K. 2002. Chthamalus montagui Montagu's stellate barnacle. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1322.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2002-01-28 Montagu's stellate barnacle (Chthamalus montagui) - Marine Life Information Network See online review for distribution map Close up of Chthamalus montagui from High Water of Spring Tide level seen dry. -

Balanus Trigonus
Nauplius ORIGINAL ARTICLE THE JOURNAL OF THE Settlement of the barnacle Balanus trigonus BRAZILIAN CRUSTACEAN SOCIETY Darwin, 1854, on Panulirus gracilis Streets, 1871, in western Mexico e-ISSN 2358-2936 www.scielo.br/nau 1 orcid.org/0000-0001-9187-6080 www.crustacea.org.br Michel E. Hendrickx Evlin Ramírez-Félix2 orcid.org/0000-0002-5136-5283 1 Unidad académica Mazatlán, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México. A.P. 811, Mazatlán, Sinaloa, 82000, Mexico 2 Oficina de INAPESCA Mazatlán, Instituto Nacional de Pesca y Acuacultura. Sábalo- Cerritos s/n., Col. Estero El Yugo, Mazatlán, 82112, Sinaloa, Mexico. ZOOBANK http://zoobank.org/urn:lsid:zoobank.org:pub:74B93F4F-0E5E-4D69- A7F5-5F423DA3762E ABSTRACT A large number of specimens (2765) of the acorn barnacle Balanus trigonus Darwin, 1854, were observed on the spiny lobster Panulirus gracilis Streets, 1871, in western Mexico, including recently settled cypris (1019 individuals or 37%) and encrusted specimens (1746) of different sizes: <1.99 mm, 88%; 1.99 to 2.82 mm, 8%; >2.82 mm, 4%). Cypris settled predominantly on the carapace (67%), mostly on the gastric area (40%), on the left or right orbital areas (35%), on the head appendages, and on the pereiopods 1–3. Encrusting individuals were mostly small (84%); medium-sized specimens accounted for 11% and large for 5%. On the cephalothorax, most were observed in branchial (661) and orbital areas (240). Only 40–41 individuals were found on gastric and cardiac areas. Some individuals (246), mostly small (95%), were observed on the dorsal portion of somites. -

Title a REVISION of the DEEP-SEA BARNACLES PACHYLASMA AND
A REVISION OF THE DEEP-SEA BARNACLES PACHYLASMA AND HEXELASMA FROM JAPAN, WITH Title A PROPOSAL OF NEW CLASSIFICATION OF THE CHTHAMALIDAE (CIRRIPEDIA, THORACICA) Author(s) Utinomi, Huzio PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1968), 16(1): 21-39 Issue Date 1968-06-29 URL http://hdl.handle.net/2433/175492 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University A REVISION OF THE DEEP-SEA BARNACLES P ACHYLASMA AND HEXELASMA FROM JAPAN, WITH A PROPOSAL OF NEW CLASSIFICATION OF THE CHTHAMALIDAE (CIRRIPEDIA, THORACICA)') Huz10 UTINOMI Seto Marine Biological Laboratory, Sirahama With 7 Text-figures SYNOPSIS The deep-sea barnacles Pachylasma and Hexelasma are little known and a few species have been sporadically recorded from isolated localities of all oceans. Of the genus Pachylasma, only two species P. crinoidophilum PrLSBRY and P.japonicum HrRo have been known from Japan. A third species P. scutistriatum BROCH is newly added to the Japanese fauna. The other known species, P. ecaudatum HrRo formerly referred to Pachylasma, is now transferred to the genus Hexelasma, of which two species H. velutinum HoEK and H. callistoderma PILSBRY only have been so far known from Japan. An attempt to subdivide the family Chthamalidae into three subfamilies (Catophragminae, Chthamalinae and Pachylasminae) is newly presented. Syste~natic Account of the Japanese Species (Revised) Genus Pachylasma DARWIN, 1854 Diagnosis. Chthamalidae having a wall of eight compartments, in which the rostrum and rostrolaterals are united by inconspicuous, linear sutures, or are wholly con crescent in the adult stage, the wall thus becoming virtually six-plated. Radii wanting or very narrow and not well differentiated from the parietes. -

South Carolina Department of Natural Resources
FOREWORD Abundant fish and wildlife, unbroken coastal vistas, miles of scenic rivers, swamps and mountains open to exploration, and well-tended forests and fields…these resources enhance the quality of life that makes South Carolina a place people want to call home. We know our state’s natural resources are a primary reason that individuals and businesses choose to locate here. They are drawn to the high quality natural resources that South Carolinians love and appreciate. The quality of our state’s natural resources is no accident. It is the result of hard work and sound stewardship on the part of many citizens and agencies. The 20th century brought many changes to South Carolina; some of these changes had devastating results to the land. However, people rose to the challenge of restoring our resources. Over the past several decades, deer, wood duck and wild turkey populations have been restored, striped bass populations have recovered, the bald eagle has returned and more than half a million acres of wildlife habitat has been conserved. We in South Carolina are particularly proud of our accomplishments as we prepare to celebrate, in 2006, the 100th anniversary of game and fish law enforcement and management by the state of South Carolina. Since its inception, the South Carolina Department of Natural Resources (SCDNR) has undergone several reorganizations and name changes; however, more has changed in this state than the department’s name. According to the US Census Bureau, the South Carolina’s population has almost doubled since 1950 and the majority of our citizens now live in urban areas. -

Do Storms Trigger Synchronous Larval Release in Semibalanus Balanoides?
Mar Biol (2011) 158:1581–1589 DOI 10.1007/s00227-011-1671-1 ORIGINAL PAPER High-frequency observations of early-stage larval abundance: do storms trigger synchronous larval release in Semibalanus balanoides? Joanna Gyory • Jesu´s Pineda Received: 12 April 2010 / Accepted: 10 March 2011 / Published online: 24 March 2011 Ó Springer-Verlag 2011 Abstract The acorn barnacle, Semibalanus balanoides,is balanoides (L.) is among the coastal marine invertebrates thought to release larvae in response to phytoplankton that reproduce synchronously. It is a highly abundant, boreo- blooms, but there is evidence that another, unidentified cue arctic barnacle species found on rocky intertidal zones along for release may exist. We conducted high-frequency sam- the eastern and western coasts of North America and the pling in Little Harbor, Massachusetts, USA, to determine northwestern coast of Europe. It reproduces once per year in whether early-stage larval abundance was related to several the winter or spring. Within a population, planktonic larvae environmental variables, and to characterize vertical dis- are released in one or more bursts, and the release period tributions of the larvae. Larval concentrations peaked at generally ends in less than 2 weeks (Barnes 1956; Crisp 2.52 and 1.02 individuals l-1 during two storms. Larvae 1959; Lang and Ackenhusen–Johns 1981; Pineda et al. were more abundant near the surface than near the bottom. 2004). The larvae can constitute 15% of zooplankton in We suggest the hypothesis that turbid conditions and coastal waters of the northeastern United States (Frolander upward-swimming behavior may protect newly-released 1955), but their pervasiveness is limited, since the planktonic larvae from predation and cannibalism.