Therapeutic Sirna: State of the Art
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Split Deoxyribozyme Probe for Efficient Detection of Highly Structured RNA Targets
University of Central Florida STARS Honors Undergraduate Theses UCF Theses and Dissertations 2018 Split Deoxyribozyme Probe For Efficient Detection of Highly Structured RNA Targets Sheila Raquel Solarez University of Central Florida Part of the Biochemistry Commons, and the Biology Commons Find similar works at: https://stars.library.ucf.edu/honorstheses University of Central Florida Libraries http://library.ucf.edu This Open Access is brought to you for free and open access by the UCF Theses and Dissertations at STARS. It has been accepted for inclusion in Honors Undergraduate Theses by an authorized administrator of STARS. For more information, please contact [email protected]. Recommended Citation Solarez, Sheila Raquel, "Split Deoxyribozyme Probe For Efficient Detection of Highly Structured RNA Targets" (2018). Honors Undergraduate Theses. 311. https://stars.library.ucf.edu/honorstheses/311 SPLIT DEOXYRIBOZYME PROBE FOR EFFICIENT DETECTION OF HIGHLY STRUCTURED RNA TARGETS By SHEILA SOLAREZ A thesis submitted in partial fulfillment of the requirements for the Honors in the Major Program in Biological Sciences in the College of Sciences and the Burnett Honors College at the University of Central Florida Orlando, Florida Spring Term, 2018 Thesis Chair: Yulia Gerasimova, PhD ABSTRACT Transfer RNAs (tRNAs) are known for their role as adaptors during translation of the genetic information and as regulators for gene expression; uncharged tRNAs regulate global gene expression in response to changes in amino acid pools in the cell. Aminoacylated tRNAs play a role in non-ribosomal peptide bond formation, post-translational protein labeling, modification of phospholipids in the cell membrane, and antibiotic biosynthesis. [1] tRNAs have a highly stable structure that can present a challenge for their detection using conventional techniques. -

Mrna Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection
International Journal of Molecular Sciences Review mRNA Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection 1, 1, 2 1,3, Shuqin Xu y, Kunpeng Yang y, Rose Li and Lu Zhang * 1 State Key Laboratory of Genetic Engineering, Institute of Genetics, School of Life Science, Fudan University, Shanghai 200438, China; [email protected] (S.X.); [email protected] (K.Y.) 2 M.B.B.S., School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China; [email protected] 3 Shanghai Engineering Research Center of Industrial Microorganisms, Shanghai 200438, China * Correspondence: [email protected]; Tel.: +86-13524278762 These authors contributed equally to this work. y Received: 30 July 2020; Accepted: 30 August 2020; Published: 9 September 2020 Abstract: Messenger ribonucleic acid (mRNA)-based drugs, notably mRNA vaccines, have been widely proven as a promising treatment strategy in immune therapeutics. The extraordinary advantages associated with mRNA vaccines, including their high efficacy, a relatively low severity of side effects, and low attainment costs, have enabled them to become prevalent in pre-clinical and clinical trials against various infectious diseases and cancers. Recent technological advancements have alleviated some issues that hinder mRNA vaccine development, such as low efficiency that exist in both gene translation and in vivo deliveries. mRNA immunogenicity can also be greatly adjusted as a result of upgraded technologies. In this review, we have summarized details regarding the optimization of mRNA vaccines, and the underlying biological mechanisms of this form of vaccines. Applications of mRNA vaccines in some infectious diseases and cancers are introduced. It also includes our prospections for mRNA vaccine applications in diseases caused by bacterial pathogens, such as tuberculosis. -

Patenting Interfering RNA
Patenting Interfering RNA J. Douglas Schultz SPE Art Unit 1635 (571) 272-0763 [email protected] Oligonucleotide Inhibitors: Mechanisms of Action RNAi - Mechanism of Action • dsRNA induces sequence-specific degradation of homologous gene transcripts • RISC metabolizes dsRNA to small 21-23- nucleotide siRNAs – RISC contains dsRNase (“Dicer”), ssRNase (Argonaut 2 or Ago2) • RISC utilizes antisense strand as “guide” to find cleavable target siRNA Mechanism of Action miRNA Mechanism of Action Interfering RNA Glossary of Terms • RNAi – RNA interference • dsRNA – double stranded RNA • siRNA – small interfering RNA, double stranded, 21-23 nucleotides • shRNA – short hairpin RNA (doubled stranded by virtue of a ssRNA folding back on itself) • miRNA – micro RNA • RISC – RNA-induced silencing complex – Dicer – RNase endonuclease siRNA miRNA • Exogenously delivered • Endogenously produced • 21-23mer dsRNA • 21-23mer dsRNA • Acts through RISC • Acts through RISC • Induces homologous target • Induces homologous target cleavage cleavage • Perfect sequence match • Imperfect sequence match – Results in target degradation – Results in translation arrest RNAi Patentability issues Sample Claims: • A siRNA that inhibits expression of a nucleic acid encoding protein X. OR • A siRNA comprising a 2’-modification, wherein said modification comprises 2’-fluoro, 2’-O-methyl, or 2’- deoxy. (Note: no target recited) OR • A method of reducing tumor cell growth comprising administering siRNA targeting protein X. RNAi Patentability Issues 35 U.S.C. 101 – Utility • Credible/Specific/Substantial/Well Established. • Used to attempt modulation of gene expression in human diseases • Routinely investigate gene function in a high throughput fashion or to (see Rana RT, Nat. Rev. Mol. Cell Biol. 2007, Vol. 8:23-36). -

(12) Patent Application Publication (10) Pub. No.: US 2004/0086860 A1 Sohail (43) Pub
US 20040O86860A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0086860 A1 Sohail (43) Pub. Date: May 6, 2004 (54) METHODS OF PRODUCING RNAS OF Publication Classification DEFINED LENGTH AND SEQUENCE (51) Int. Cl." .............................. C12Q 1/68; C12P 19/34 (76) Inventor: Muhammad Sohail, Marston (GB) (52) U.S. Cl. ............................................... 435/6; 435/91.2 Correspondence Address: MINTZ, LEVIN, COHN, FERRIS, GLOWSKY (57) ABSTRACT AND POPEO, PC. ONE FINANCIAL CENTER Methods of making RNA duplexes and single-stranded BOSTON, MA 02111 (US) RNAS of a desired length and Sequence based on cleavage of RNA molecules at a defined position, most preferably (21) Appl. No.: 10/264,748 with the use of deoxyribozymes. Novel deoxyribozymes capable of cleaving RNAS including a leader Sequence at a (22) Filed: Oct. 4, 2002 Site 3' to the leader Sequence are also described. Patent Application Publication May 6, 2004 Sheet 1 of 2 US 2004/0086860 A1 DNA Oligonucleotides T7 Promoter -TN-- OR 2N-2-N-to y Transcription Products GGGCGAAT-N-UU GGGCGAAT-N-UU w N Deoxyribozyme Cleavage - Q GGGCGAAT -------' Racction GGGCGAAT N-- UU N-UU ssRNA products N-UU Anneal ssRNA UU S-2N- UU siRNA product FIGURE 1: Flowchart summarising the procedure for siRNA synthesis. Patent Application Publication May 6, 2004 Sheet 2 of 2 US 2004/0086860 A1 Full-length transcript 3'-digestion product 5'-digestion product (5'GGGCGAATA) A: Production of single-stranded RNA templates by in vitro transcription and digestion With a deoxyribozyme V 2- 2 V 22inv 22 * 2 &3 S/AS - 88.8x, *...* or as IGFR -- is as 4. -

Expanding the Genetic Code Lei Wang and Peter G
Reviews P. G. Schultz and L. Wang Protein Science Expanding the Genetic Code Lei Wang and Peter G. Schultz* Keywords: amino acids · genetic code · protein chemistry Angewandte Chemie 34 2005 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim DOI: 10.1002/anie.200460627 Angew. Chem. Int. Ed. 2005, 44,34–66 Angewandte Protein Science Chemie Although chemists can synthesize virtually any small organic molecule, our From the Contents ability to rationally manipulate the structures of proteins is quite limited, despite their involvement in virtually every life process. For most proteins, 1. Introduction 35 modifications are largely restricted to substitutions among the common 20 2. Chemical Approaches 35 amino acids. Herein we describe recent advances that make it possible to add new building blocks to the genetic codes of both prokaryotic and 3. In Vitro Biosynthetic eukaryotic organisms. Over 30 novel amino acids have been genetically Approaches to Protein encoded in response to unique triplet and quadruplet codons including Mutagenesis 39 fluorescent, photoreactive, and redox-active amino acids, glycosylated 4. In Vivo Protein amino acids, and amino acids with keto, azido, acetylenic, and heavy-atom- Mutagenesis 43 containing side chains. By removing the limitations imposed by the existing 20 amino acid code, it should be possible to generate proteins and perhaps 5. An Expanded Code 46 entire organisms with new or enhanced properties. 6. Outlook 61 1. Introduction The genetic codes of all known organisms specify the same functional roles to amino acid residues in proteins. Selectivity 20 amino acid building blocks. These building blocks contain a depends on the number and reactivity (dependent on both limited number of functional groups including carboxylic steric and electronic factors) of a particular amino acid side acids and amides, a thiol and thiol ether, alcohols, basic chain. -
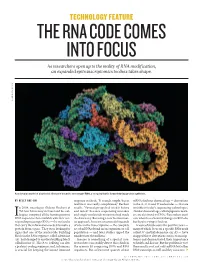
THE RNA CODE COMES INTO FOCUS As Researchers Open up to the Reality of RNA Modification, an Expanded Epitranscriptomics Toolbox Takes Shape
TECHNOLOGY FEATURE THE RNA CODE COMES INTO FOCUS As researchers open up to the reality of RNA modification, an expanded epitranscriptomics toolbox takes shape. LAGUNA DESIGN/SPL LAGUNA A molecular model of a bacterial ribosome bound to messenger RNA, a complex that is formed during protein synthesis. BY KELLY RAE CHI response in check. “It sounds simple, but in mRNAs harbour chemical tags — decorations real life it was really complicated,” Rechavi to the A, C, G and U nucleotides — that are n 2004, oncologist Gideon Rechavi at recalls. “Several groups had tried it before invisible to today’s sequencing technologies. Tel Aviv University in Israel and his col- and failed” because sequencing mistakes (Similar chemical tags, called epigenetic mark- leagues compared all the human genomic and single-nucleotide mutations had made ers, are also found on DNA.) Researchers aren’t IDNA sequences then available with their cor- the data noisy. But using a new bioinformat- sure what these chemical changes in RNA do, responding messenger RNAs — the molecules ics approach, his team uncovered thousands but they’re trying to find out. that carry the information needed to make a of sites in the transcriptome — the complete A wave of studies over the past five years — protein from a gene. They were looking for set of mRNAs found in an organism or cell many of which focus on a specific RNA mark signs that one of the nucleotide building population — and later studies upped the called N6-methyladenosine (m6A) — have blocks in the RNA sequence, called adenosine number into the millions1. -

Small Interfering RNA-Mediated Translation Repression Alters Ribosome Sensitivity to Inhibition by Cycloheximide in Chlamydomonas Reinhardtii
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Dissertations and Theses in Biological Sciences Biological Sciences, School of Spring 2013 Small Interfering RNA-Mediated Translation Repression Alters Ribosome Sensitivity to Inhibition by Cycloheximide in Chlamydomonas reinhardtii Xinrong Ma University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/bioscidiss Part of the Biology Commons, Cellular and Molecular Physiology Commons, Microbiology Commons, and the Molecular Genetics Commons Ma, Xinrong, "Small Interfering RNA-Mediated Translation Repression Alters Ribosome Sensitivity to Inhibition by Cycloheximide in Chlamydomonas reinhardtii" (2013). Dissertations and Theses in Biological Sciences. 51. https://digitalcommons.unl.edu/bioscidiss/51 This Article is brought to you for free and open access by the Biological Sciences, School of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Dissertations and Theses in Biological Sciences by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. SMALL INTERFERING RNA-MEDIATED TRANSLATION REPRESSION ALTERS RIBOSOME SENSITIVITY TO INHIBITION BY CYCLOHEXIMIDE IN CHLAMYDOMONAS REINHARDTII by Xinrong Ma A DISSERTATION Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Doctor of Philosophy Major: Biological Sciences Under the Supervision of Professor Heriberto Cerutti Lincoln, Nebraska May, 2013 SMALL INTERFERING RNA-MEDIATED TRANSLATION REPRESSION ALTERS RIBOSOME SENSITIVITY TO INHIBITION BY CYCLOHEXIMIDE IN CHLAMYDOMONAS REINHARDTII Xinrong Ma, Ph.D. University of Nebraska, 2013 Advisor: Heriberto Cerutti RNA interference (RNAi) is an evolutionarily conserved gene silencing mechanism in eukaryotes, with regulatory roles in a variety of biological processes, including cell cycle, cell differentiation, physiological and metabolic pathways, and stress responses. -

Mrna, Rrna and Trna Types of RNA: Mrna, Rrna and Trna
Types of RNA: mRNA, rRNA and tRNA Types of RNA: mRNA, rRNA and tRNA By Susha Cheriyedath, M.Sc. Reviewed by Michael Greenwood, M.Sc. RNA or ribonucleic acid is a polymer of nucleotides that is made up of a ribose sugar, a phosphate, and bases such as adenine, guanine, cytosine, and uracil. It plays a crucial role in gene expression by acting as the intermediate between the genetic information encoded by DNA and proteins. Designua | Shutterstock RNA has a structure very similar to that of DNA. The key difference in RNA structure is that the ribose sugar in RNA possesses a hydroxyl (OH) group that is absent in DNA. Types of RNA In both prokaryotes and eukaryotes, there are three main types of RNA – messenger RNA (mRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA). These 3 types of RNA are discussed below. P Saved from URL: https://www.news-medical.net/life-sciences/-Types-of-RNA-mRNA-rRNA-and-tRNA.aspx 1/5 Types of RNA: mRNA, rRNA and tRNA Messenger RNA (mRNA) mRNA accounts for just 5% of the total RNA in the cell. mRNA is the most heterogeneous of the 3 types of RNA in terms of both base sequence and size. It carries complimentary genetic code copied, from DNA during transcription, in the form of triplets of nucleotides called codons. Each codon specifies a particular amino acid, though one amino acid may be coded for by many different codons. Although there are 64 possible codons or triplet bases in the genetic code, only 20 of them represent amino acids. -

A Study on Recent Implication of Nanotechnology in Drug Delivery Systems
Available online a t www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2015, 7 (7):213-220 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4 A study on recent implication of nanotechnology in drug delivery systems Mahendra Pratap Singh 1, Parjanya Kumar Shukla 1,2* , Amita Verma 2 and Ramesh Patel 1 1Krishnarpit Institute of Pharmacy, Allahabad 2Department of Pharmaceutical Sciences, Faculty of Health Science, Sam Higginbottom Institute of Agriculture, Technology & Sciences, Allahabad, India _____________________________________________________________________________________________ ABSTRACT Efficient drug targeting to diseases by circumventing all the shortcomings of conventional drug delivery systems can be achieved by the significant approach of advances in nanotechnology. Nanotechnology will affect our lives tremendously over the next decade in very different fields, including medicine and pharmaceutical Sciences. Most of the available drugs now are lipophilic in nature and this stands as challenging aspect faced for scientists to formulate and deliver for better efficacy, so nanoparticles, nanosuspension, nanocapsules are used now days to deliver these drugs with greater bioavailability and also have been adopted to improve the solubility of poorly soluble drugs. The use of nanoparticles is an universal formulation approach to increase the therapeutic performance of drugs in any route of administration. This review article describes the preparation methods, physicochemical -

IGA 8/E Chapter 8
8 RNA: Transcription and Processing WORKING WITH THE FIGURES 1. In Figure 8-3, why are the arrows for genes 1 and 2 pointing in opposite directions? Answer: The arrows for genes 1 and 2 indicate the direction of transcription, which is always 5 to 3. The two genes are transcribed from opposite DNA strands, which are antiparallel, so the genes must be transcribed in opposite directions to maintain the 5 to 3 direction of transcription. 2. In Figure 8-5, draw the “one gene” at much higher resolution with the following components: DNA, RNA polymerase(s), RNA(s). Answer: At the higher resolution, the feathery structures become RNA transcripts, with the longer transcripts occurring nearer the termination of the gene. The RNA in this drawing has been straightened out to illustrate the progressively longer transcripts. 3. In Figure 8-6, describe where the gene promoter is located. Chapter Eight 271 Answer: The promoter is located to the left (upstream) of the 3 end of the template strand. From this sequence it cannot be determined how far the promoter would be from the 5 end of the mRNA. 4. In Figure 8-9b, write a sequence that could form the hairpin loop structure. Answer: Any sequence that contains inverted complementary regions separated by a noncomplementary one would form a hairpin. One sequence would be: ACGCAAGCUUACCGAUUAUUGUAAGCUUGAAG The two bold-faced sequences would pair and form a hairpin. The intervening non-bold sequence would be the loop. 5. How do you know that the events in Figure 8-13 are occurring in the nucleus? Answer: The figure shows a double-stranded DNA molecule from which RNA is being transcribed. -

A Gellan-Based Fluid Gel Carrier for Spray Delivery
AGELLAN-BASEDFLUIDGELCARRIERFOR SPRAY DELIVERY by BRITT TER HORST Athesissubmittedto The University of Birmingham for the degree of DOCTOR OF PHILOSOPHY School of Chemical Engineering College of Engineering and Physical Sciences The University of Birmingham May 2019 UNIVERSITYDF BIRMINGHAM University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. Abstract Autologous cell transplantation is a promising approach to enhance burn wound re- epithelialisation. It was introduced to clinical practice decades ago with current delivery techniques involving spraying autologous cultured or uncultured cells in low-viscosity sus- pensions. This delivery method is limited since it results in an uneven distribution on the wound bed and cell loss as the liquid is not retained on the skin surface. In this thesis, a sprayable gel that solidifies on the surface of the skin has been developed to circumvent this problem. A gellan-based fluid gel system was developed with flexible viscoelastic properties that can be tuned by a biocompatible polymer concentration and ionic strength to facilitate spray delivery. The material liquefies at high shear during spraying with self-healing properties of the gel causing it to solidify on the receiving sur- face. -

Powders Are Intimate Mixtures of Dry, Finely Divided Drugs And/Or Chemicals That May Be Intended for Internal Or External Use
Powders are intimate mixtures of dry, finely divided drugs and/or chemicals that may be intended for internal or external use. Advantages/Disadvantages flexibility in compounding good chemical stability Ease of administration time-consuming not well-suited to dispense unpleasant tasting, hygroscopic or deliquescent drugs inaccuracy of dose Types Medicated Aerosol Preparation Weighing of ingredients Comminuting Blending or mixing Weighing of individual dose Materials Active ingredients Packaging Types of material used to pack/dispense divided powders Foil plastic bag used for volatile vegetable parchment thin, semiopaque, moisture-resistant white bond without moisture-resistant properties Glassine glazed, transparent, moisture-resistant paper used for volatile to a certain extent Waxed transparent-water-proof paper used for hygroscopic and volatile drugs Seidlitz powder is the name with which is commonly known a medication composed by a mixture of tartaric acid, sodium bicarbonate, and potassium sodium tartrate, used as a mild cathartic by dissolving in water and drinking. After ingestion, the powder combines with gastric juices developing intestinal gases which are somewhat helpful in evacuating the bowels. This medication's name comes from the Seidlitz Saline Springs of Bohemia (now Sedlčany in the Czech Republic), which were rather famous in Europe at the time this medication was first marketed in the late 19th century, even though the foregoing laxative constituents do not represent those of the springs named. Use of Each ingredient – act as acid and base which react in the presence of water to cause effervescence Use of Preparation - mild cathartic/laxative Appearance – white powder Storage – store in air tight containers Granules are prepared agglomerates of powdered materials, may be used per se for the medicinal value of their content or they may be used for pharmaceutical purposes, as in making tablets.