Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation Reactions of Substituted Ketenes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Chemistry
Wisebridge Learning Systems Organic Chemistry Reaction Mechanisms Pocket-Book WLS www.wisebridgelearning.com © 2006 J S Wetzel LEARNING STRATEGIES CONTENTS ● The key to building intuition is to develop the habit ALKANES of asking how each particular mechanism reflects Thermal Cracking - Pyrolysis . 1 general principles. Look for the concepts behind Combustion . 1 the chemistry to make organic chemistry more co- Free Radical Halogenation. 2 herent and rewarding. ALKENES Electrophilic Addition of HX to Alkenes . 3 ● Acid Catalyzed Hydration of Alkenes . 4 Exothermic reactions tend to follow pathways Electrophilic Addition of Halogens to Alkenes . 5 where like charges can separate or where un- Halohydrin Formation . 6 like charges can come together. When reading Free Radical Addition of HX to Alkenes . 7 organic chemistry mechanisms, keep the elec- Catalytic Hydrogenation of Alkenes. 8 tronegativities of the elements and their valence Oxidation of Alkenes to Vicinal Diols. 9 electron configurations always in your mind. Try Oxidative Cleavage of Alkenes . 10 to nterpret electron movement in terms of energy Ozonolysis of Alkenes . 10 Allylic Halogenation . 11 to make the reactions easier to understand and Oxymercuration-Demercuration . 13 remember. Hydroboration of Alkenes . 14 ALKYNES ● For MCAT preparation, pay special attention to Electrophilic Addition of HX to Alkynes . 15 Hydration of Alkynes. 15 reactions where the product hinges on regio- Free Radical Addition of HX to Alkynes . 16 and stereo-selectivity and reactions involving Electrophilic Halogenation of Alkynes. 16 resonant intermediates, which are special favor- Hydroboration of Alkynes . 17 ites of the test-writers. Catalytic Hydrogenation of Alkynes. 17 Reduction of Alkynes with Alkali Metal/Ammonia . 18 Formation and Use of Acetylide Anion Nucleophiles . -

Chip Incompatibility Filters
ChIP Incompatibility Filters Filter Name Type Description includes carboxylic acid halides and >1 acyl halide and related SMARTS derivatives like chloroformates, carbamoyl- , imidoyl halides, etc. >1 aldehyde SMARTS R no heteroatom no isocyanate, ketene, etc. >1 alkyl bromide / iodide SMARTS no acyl halide or related or vinyl halide >1 amine aromatic primary SMARTS aromatic carbon bound to N, N not charged >1 amines (aromatic/aliphatic, primary no amide, enamine, etc., no heteroatom SMARTS or secondary) bound to N, N not charged no amide, enamine, etc, no heteroatom >1 amines nucleophilic (aliphatic SMARTS bound to N, no aromatic carbon bound to primary or secondary) N, N not charged >1 aryl bromide / iodide SMARTS any aryl bromide / iodide >1 aryl halide SMARTS any aryl halide any boronic acid derivative, aromatic or >1 boronic acid derivative SMARTS aliphatic >1 carbonyl acid SMARTS any carboxylic or carbamic acid, etc. >1 carboxylic acid anhydrides SMARTS carbon must be bound to carbonyl no heteroatom bound to carbonyl or >1 carboxylic acid ester SMARTS oxygen, no acid, no anydride, etc >1 isocyanate / isothiocyanate SMARTS no restrictions to nitrogen substituents R no heteroatom, no isocyanate, ketene, >1 ketone or aldehyde SMARTS etc. >1 NH any SMARTS R can be anything >1 thioamide and related (any) SMARTS any substitution >1 thiol and related (nucleophic) SMARTS any SH or negative S >2 NH any SMARTS R can be anything acidic compounds I combination sulfonyl acids and carboxylic acids anhydrides, bicarbonates, thio and imino acyl anhydrides and derivatives SMARTS derivatives, etc. includes carboxylic acid halides and acyl halide and related SMARTS derivatives like chloroformates, carbamoyl- , imidoyl halides, etc. -

United States Patent Office
Patented Dec. 23, 1947 2432,991 UNITED STATES PATENT OFFICE ACYLATION OF THOPHENE Howard D. Hartough, Pitman, and John J. Sar della, Woodbury, N. J., assigners to Socony Wacuum Oil Company, incorporated, a corpo ration of New York No Drawing. Application January 8, 1946, Seria No. 642.13 12 Claims, (C. 260-329) 2 This invention relates to a catalytic acylation in carbon disulfide to a suspension of aluminum process for thiophenes and, more particularly, is chloride in the same solvent. If, however, a car directed to a method for acylating thiophene and oon disulfide solution of the acid chloride was its derivatives in the presence of glauconite as a added to a suspension of thiophene and alumi catalyst. r nun chloride, much tar was formed and a low The acylation of thiophene. and thiophene de yield of ketone resulted. The acylation of thio rivatives has previously been carried out employ phene has, accordingly, been an exceedingly dif ing Organic acid anhydrides, acyl halides, and fictly reaction to carry out, the usual acylation acyl nitriles as acylating agents and in the pres catalysts causing excessive resinification of the eace of various catalysts, including aluminun thiophene reactant. The resinification usually chloride, stannic chloride, titanium tetrachloride, occurs before acylation can be effected, and if phosphorus pentoxide and 2-chloronercurithio 'the expected reaction product is formed, it is phene. Other methodis of inaking acylated thio generally only in relatively Small amounts. phene include the dry distillation of calcium It has now been discovered that acylated thio salts of thiophene carboxylic acids and the action s phenes may be obtained in an efficient manner of nitriles on thienyiragnesium iodide. -

C:\Documents and Settings\Steve Murov\My
125 Exercise 15 - Preparation of a Key for Reaction-Map of Organic Chemistry (see Appendix C in student manual) Abstract: The Reaction -Map of Organic Chemistry has been designed to give organic chemistry students an overview of most of the reactions needed for the organic chemistry course. The chart has been partially organized according to the periodic table on the horizontal axis and according to carbon oxidation level on the vertical axis. In addition the carboxyls are grouped vertically according to decreasing reactivity and carbon - carbon bond forming reactions are emphasized with bold arrows. The chart provides a study aide for students and should help students develop synthetic routes from one functional group to another. The chart should be especially useful for students studying for the final examination for the two semester organic chemistry course. In addition to the chart, three keys are available that organize the reactions according to mechanism, functional group preparations and functional group reactions. Chemistry can be thought of a search for order in matter and this chart attempts to provide some insight into the order that exists in organic chemistry. Please note, “Supporting information reprinted with permission from J. Chem. Ed. 2007, 84(7), 1224. Copyright 2007 American Chemical Society.” Reaction-Map as a Study Aid At the end of a two semester course in organic chemistry, a student should be able to perform the exercises below. (Note: In addition to the exercises below, a student of organic chemistry should be able to demonstrate competency with spectroscopic, stereochemical and multistep synthetic challenges.) By performing the exercises below should result in the preparation of three keys for the Reaction-Map of Organic Chemistry. -

Diblock Copolymers Via Atom Transfer Radical Polymerization Utilizing Halide Exchange Technique
Polymer Journal, Vol. 37, No. 2, pp. 102–108 (2005) Synthesis of Amphiphilic Poly(ethylene oxide)-b-Poly(methyl methacrylate) Diblock Copolymers via Atom Transfer Radical Polymerization Utilizing Halide Exchange Technique y Xiaoyi SUN,1 Hailiang ZHANG,1; Lingjun ZHANG,1 Xiayu WANG,1 and Qi-Feng ZHOU2 1Institute of Polymer Science, Xiangtan University, Xiangtan 411105, Hunan Province, China 2Department of Polymer Science and Engineering, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China (Received September 14, 2004; Accepted November 17, 2004; Published February 15, 2005) ABSTRACT: Amphiphilic diblock copolymers with different molecular weights and low polydispersities were synthesized by atom transfer radical polymerization (ATRP) of methyl methacrylate (MMA) using PEO–Br as an ini- tiator, which was obtained by the esterification of poly(ethylene oxide) (PEO) with 2-bromoisobutyryl bromide. The polymerization proceeded in solution using halide exchange technique to control ATRP. Fourier transform infrared spectroscopy (FT-IR) and 1H NMR studies confirm the composition of PEO–Br macroinitiator and related diblock co- polymers. The results obtained by GPC analysis show that the number average molecular weight was increased versus monomer conversion and the polydispersities were quite low (<1:10), which is the character of a controlled/‘‘living’’ polymerization. Moreover, the crystallization behavior of PEO-b-PMMA block copolymers was studied by means of differential scanning calorimetry (DSC). It was found -

A Process for Preparing an Acyl Halide Or Sulfonyl Halide
~™ llll III II II III II III I II II III (19) J European Patent Office Office europeen des brevets (1 1 ) EP0 751 131 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int. CI.6: C07D 233/18, C07C 51/60, 02.01.1997 Bulletin 1997/01 C07C 303/02, C07B 41/1 0 (21) Application number: 96108936.4 (22) Date of filing: 04.06.1996 (84) Designated Contracting States: • Hayashi, Hidetoshi DE FR IT NL Ohmuta-shi, Fukuoka-ken, 836 (JP) • Mizuta, Hideki (30) Priority: 20.06.1995 JP 152826/95 Ohmuta-shi, Fukuoka-ken, 837 (JP) (71) Applicant: MITSUI TOATSU CHEMICALS, Inc. (74) Representative: Strehl Schubel-Hopf Groening & Chiyoda-Ku Tokyo 1 00 (JP) Partner Maximilianstrasse 54 (72) Inventors: 80538 Munchen (DE) • Nagata, Teruyuki Ohmuta-shi, Fukuoka-ken, 836 (JP) (54) A process for preparing an acyl halide or sulfonyl halide (57) A preparation process of acyl halide or sulfonyl halide which comprises reacting corresponding carboxylic acid or sulfonic acid with a haloiminium salt represented by the general formula (1): R'-N -R2 X ( 1 ) XCrU/n wherein R1 and R2 are same or different lower alkyl groups, X is a halogen atom, and n is an integer of 2 or 3. CO LO o Q_ LU Printed by Rank Xerox (UK) Business Services 2.13.10/3.4 EP0 751 131 A1 Description 1 . Field of the Invention 5 The present invention relates to a preparation process of acyl halide or sulfonyl halide. 2. Description of the Related Art In recent years, acyl halide has become important in industry as an intermediate for preparing heat resistant resin, 10 medicines and agricultural chemicals. -

19.2 Preparation of Acyl Chlorides
Hornback_Ch19_803-857 12/16/04 11:51 AM Page 808 808 CHAPTER 19 I SUBSTITUTIONS AT THE CARBONYL GROUP PROBLEM 19.2 Click Coached Tutorial Problems Explain whether these equilibria favor the reactants or the products: for more practice using Table O 19.1 to predict the position of X the Equilibrium in OH OCCH3 Carbonyl Group O O O Substitutions. X X X a) CH3COCCH3 ϩϩCH3COH O O X X b) CH3CH2CNH2 ϩ CH3OH CH3CH2COCH3 ϩ NH3 O O X X C± C± Cl NHCH2CH3 c) ϩϩCH3CH2NH2 HCl PROBLEM 19.3 Explain which of these reactions is faster: O O X _ X _ CH3COCH3 ϩ OH CH3CO ϩ CH3OH or O O X _ X _ CH3CNHCH3 ϩ OH CH3CO ϩ CH3NH2 PROBLEM 19.4 Suggest a reaction that could be used to prepare this amide: O X CH3CH2CH2CNHCH2CH2CH3 19.2 Preparation of Acyl Chlorides Because they are readily available from a number of synthetic reactions, carboxylic acids are the most common starting materials for the preparation of the other members of this family. Conversion of a carboxylic acid to an acyl chloride provides access to any of the other derivatives because the acyl chloride is at the top of the reactivity scale. But how can the acyl chloride be prepared from the acid when the acid is lower on the re- activity scale? This can be accomplished by using an even more reactive compound to drive the equilibrium in the desired direction. The reagent that is employed in the vast majority of cases is thionyl chloride, SOCl2. -

UNITED STATES PATENT OFFICE 2,515,123 ACYLATION of FURAN Howard D
Patented July 11, 1950 2,515,23 UNITED STATES PATENT OFFICE 2,515,123 ACYLATION OF FURAN Howard D. Hartough, Pitman, N. J., assignor to Socony-Vacuum Oil Company, Incorporated, a corporation of New York No Drawing. Application October 3, 1947, Serial No. 777,853 10 Claims. (CI. 260-345) 2 This invention relates to a process for the the acylation of furan. This is probably due to acylation of furan and, more particularly, is di the fact that acyl halides form comparatively rected to a catalytic method for acylating furan stable molecular complexes with aluminum chlo and its derivatives in the presence of a Small ride and the other above mentioned catalysts, amount of boron trifluoride. thereby decreasing their catalytic effect. Acylation reactions are well known in the art While yields as high as 50 per cent of theory and connote the union between acyl radicals have been reported using an aluminum chloride and molecules of organic compounds under COn catalyst, these figures have been the exception ditions of temperature, pressure, and time Ordi rather than the rule. In general, the yields of narily referred to in the art as acylating condi O acyl furans heretofore obtained have averaged tions. The compounds thus produced represent about 35 per cent of theory. These relatively Structurally the Substitution of the original acyl Small yields were believed to be due, at least in radical for a hydrogen atom on the organic corn part, to the relatively large quantities of catalyst pound molecule. being employed, i. e., amounts of the order of As a general rule, the temperature, pressure, 5 molecular quantities with respect to reactants and time of reaction employed in acylation Opera being used. -

Recent Advances in Acyl Suzuki Cross-Coupling
catalysts Review Recent Advances in Acyl Suzuki Cross-Coupling Jonathan Buchspies and Michal Szostak * Department of Chemistry, Rutgers University, 73 Warren Street, Newark, NJ 07102, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-973-353-5329 Received: 17 December 2018; Accepted: 28 December 2018; Published: 8 January 2019 Abstract: Acyl Suzuki cross-coupling involves the coupling of an organoboron reagent with an acyl electrophile (acyl halide, anhydride, ester, amide). This review provides a timely overview of the very important advances that have recently taken place in the acylative Suzuki cross-coupling. Particular emphasis is directed toward the type of acyl electrophiles, catalyst systems and new cross-coupling partners. This review will be of value to synthetic chemists involved in this rapidly developing field of Suzuki cross-coupling as well as those interested in using acylative Suzuki cross-coupling for the synthesis of ketones as a catalytic alternative to stoichiometric nucleophilic additions or Friedel-Crafts reactions. Keywords: Suzuki cross-coupling; acyl cross-coupling; acylation; ketones; acylative cross-coupling; palladium; nickel; phosphine; N-heterocyclic carbene; Suzuki-Miyaura 1. Introduction The Suzuki cross-coupling represents the most powerful C–C bond forming reaction in organic synthesis [1]. Traditional Suzuki cross-coupling (also referred to as Suzuki–Miyaura cross-coupling) involves the coupling of an organoboron reagent with an aryl halide (pseudohalide) and is most commonly employed for the synthesis of biaryls by a C(sp2)–C(sp2) disconnection using a palladium or nickel catalyst (Figure1A) [ 2,3]. Since the initial report in 1979, many variants of the Suzuki cross-coupling have been discovered [4]. -

Hydrides As Reducing Agents
Hydrides as Reducing Agents Lithium aluminum hydride (LiAlH4) is a strong reducing agent. It will donate hydride (“H-”) to any C=O containing functional group. Examples: + 2. H3O 1. LiAlH4 (or just H2O) aldehyde primary alcohol 1. LiAlH4 2. H2O ketone secondary alcohol Hydrides as Reducing Agents Lithium aluminum hydride (LiAlH4) is a strong reducing agent. It will reduce almost any C=O containing functional group to an alcohol. Example: 1. LiAlH4 2. H2O ester One and then another equivalent equivalent adds, of H- adds, unavoidably. Reduced by LiAlH4 to an alcohol: aldehyde ketone carboxylic acid ester acyl halide Double Addition of Hydride to Carboxylic Acids and Derivatives Why? Ketones and aldehydes are more electrophilic than acids, esters and acyl halides. As soon as a ketone or aldehyde is Lone pair donation by generated, it is immediately reduced oxygen reduces partial again. positive charge on C=O carbon. Reduced by LiAlH4 to an alcohol: aldehyde ketone carboxylic acid ester acyl halide Hydrides as Reducing Agents Exception: LiAlH4 reduces amides to amines. Examples: 1. LiAlH4 Mechanism depends slightly on whether amide has an N-H or 2. H2O not. But the result is the same. 1. LiAlH4 2. H2O Reduced by LiAlH4 to an alcohol: aldehyde ketone carboxylic acid ester acyl halide Hydrides as Reducing Agents Sodium borohydride (NaBH4) is a mild reducing agent. It is only capable of reducing aldehydes and ketones. NaBH4 NaBH4 isn’t as basic as LiAlH4, EtOH so reaction can be conducted in protic solvent, and separate workup step isn’t essential. aldehyde primary alcohol NaBH4 EtOH Reduced by NaBH4: ketone secondary alcohol aldehyde ketone Biological Cofactors as Redox Agents LiAlH4 isn’t used in biology, but biological reductants are mechanistically similar. -
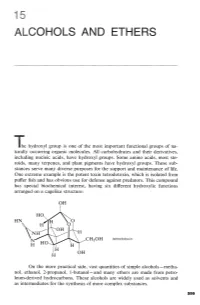
Alcohols and Ethers
ALCOHOLS AND ETHERS The hydroxyl group is one of the most important functional groups of na- turally occurring organic molecules. All carbohydrates and their derivatives, including nucleic acids, have hydroxyl groups. Some amino acids, most ste- roids, many terpenes, and plant pigments have hydroxyl groups. These sub- stances serve many diverse purposes for the support and maintenance of life. One extreme example is the potent toxin tetrodotoxin, which is isolated from puffer fish and has obvious use for defense against predators. This compound has special biochemical interest, having six different hydroxylic functions arranged on a cagelike structure: tetrodotoxin On the more practical side, vast quantities of simple alcohols - metha- nol, ethanol, 2-propanol, 1-butan01 - and many ethers are made from petro- leum-derived hydrocarbons. These alcohols are widely used as solvents and as intermediates for the synthesis of more complex substances. 15 Alcohols and Ethers The reactions involving the hydrogens of alcoholic OH groups are expected to be similar to those of water, HOH, the simplest hydroxylic com- pound. Alcohols, ROH, can be regarded in this respect as substitution products of water. However, with alcohols we shall be interested not only in reactions that proceed at the 8-H bond but also with processes that result in cleavage of the C-0 bond, or changes in the organic group R. The simple ethers, ROR, do not have 0-H bonds, and most of their reactions are limited to the substituent groups. The chemistry of ethers, there- fore, is less varied than that of alcohols. This fact is turned to advantage in the widespread use of ethers as solvents for a variety of organic reactions, as we already have seen for Grignard reagents (Section 14- 10). -

Organic Chemistry – the Functional Group Approach Organic Chemistry
Organic Chemistry – The Functional Group Approach OH Br alkane alcohol halide alkene (no F.G.) non-polar (grease, fats) polar (water soluble) non-polar (water insoluble) non-polar (water insoluble) tetrahedral tetrahedral tetrahedral trigonal O NH alkyne aromatic aldehyde/ketone imine non-polar (water insoluble) non-polar (water insoluble) polar (water soluble) polar (water soluble) linear flat trigonal trigonal YSU Organic Chemistry – The Functional Group Approach NH O HO OH H3CO OCH3 2 OH hydrate acetal amine carboxylic acid polar (water soluble) non-polar (water insoluble) polar (water soluble) polar(watersoluble) tetrahedral tetrahedral tetrahedral trigonal O O O O O OCH3 NH2 Cl O carboxylic ester carboxylic amide acyl halide acid anhydride polar (water-solube) polar (water soluble) non-polar (reacts w/water) non-polar (reacts w/water) trigonal trigonal trigonal trigonal YSU 1 Organic Chemistry – The Functional Group Approach OH Br alkane alcohol halide alkene (no F.G.) non-polar (grease, fats) polar (water soluble) non-polar (water insoluble) non-polar (water insoluble) tetrahedral tetrahedral tetrahedral trigonal O NH alkyne aromatic aldehyde/ketone imine non-polar (water insoluble) non-polar (water insoluble) polar (water soluble) polar (water soluble) linear flat trigonal trigonal YSU Organic Chemistry – The Functional Group Approach OH Br alkane alcohol halide alkene (no F.G.) non-polar (grease, fats) polar (water soluble) non-polar (water insoluble) non-polar (water insoluble) tetrahedral tetrahedral tetrahedral trigonal O NH