Selected Antioxidants in Organic Vs. Conventionally Grown Apple Fruits
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sheriff Investigates Royalton Homicide
PINE CITY THURSDAY, MARCH 12, 2020 PIONEER VOL. 135 NO. 11 www.pinecitymn.com $1.00 CAMS FOR DEPUTIES: Pine County Sheriff’s Office makes plans for body cameras.P7 Sheriff investigates Royalton homicide STAFF REPORT sion was called in to assist in the [email protected] investigation. The man was transported to The Pine County Sheriff’s the Midwest Medical Examiner’s Office is investigating what is Office for autopsy. being called a homicide in Royal- The deceased male has been ton Township. identified as: Scott A. Ness, 61, According to the Sheriff’s no permanent address. Investi- Office, on March 2 at 10:09 a.m. gators are working on a time- dispatchers received a call about line surrounding his death and a possible deceased person at a following up on any leads. property on Royal Heights Lane Anyone with information in Royalton Township. about this case is asked to con- Deputies responded to the tact the Pine County Sheriff’s scene and found a man who was Office at 320-629-8380. Tips can obviously deceased inside of also be sent to: investigators@ a motorhome. The Minnesota co.pine.mn.us. Bureau of Criminal Apprehen- Pine County: A ‘Second Amendment Sanctuary?’ BY JENNIFER YOKUM-STANS to order the temporary removal [email protected] of firearms from a person who may present a danger to others A new group is forming in Pine or themselves. Refusal to comply County. Pine County For the with the order is punishable as Second Amendment is a group a criminal offense. Bills propos- of county residents looking to ing laws such as these were just make Pine County a “sanctuary passed by the House Ways and county” for second amendment Means Committee on February rights. -

Sager Farms Wins Champion Cider for Second
SCHWARTZ ORCHARDS RECLAIMS TOP HONORS AT THE 2016 ILLINOIS SWEET CIDER CONTESTS Dr. Elizabeth Wahle, Cider Contest Coordinator UI Extension Educator The Illinois State Horticulture Society sponsored its 27th annual Illinois and National Sweet Cider Contests and the 14th annual National Hard Cider Contest, held in conjunction with the Illinois Specialty Crops, Agritourism and Organic Conference on January 7th in Springfield, Illinois. Tom Schwartz of Schwartz Orchards, located at Centralia, IL, produced the No.1 overall rated cider at this year’s contest, repeating his 2014 and 2008 wins in both the National and Illinois contests. Tom pressed his winning cider for this year’s contests with a bladder press using Jonathan and Fuji apples. Second Place Illinois Cider and 2nd Place National Cider went to Joe Ringhausen of Ringhausen Orchards in Fieldon, IL. Trevor Grissom of Grissom’s Lost Creek Orchard in Greenup, IL won Third Place Illinois Cider. Tom Roney of Tuttle Orchards won 3rd Place National Cider. Midwest Cider of Merit 1st Runner-up was awarded to Raoul Bergersen of Valley Orchards in Winnebago, IL. Pat Curran of Curran’s Orchard in Rockford, IL was awarded the Midwest Cider of Merit 2nd Runner-up and the Midwest Cider of Merit 3rd Runner-up went to Craig Tanner of Tanner’s Orchard in Speer, IL. Grissom’s Lost Creek Orchard of Greenup, IL claimed the Champion Hard Cider award, making this their 5th time taking top honors in this category. Trevor Grissom produced his winning hard cider for this year’s contest with a combination of GoldRush, Golden Delicious, Gala and Jonagold apples. -

Apples Catalogue 2019
ADAMS PEARMAIN Herefordshire, England 1862 Oct 15 Nov Mar 14 Adams Pearmain is a an old-fashioned late dessert apple, one of the most popular varieties in Victorian England. It has an attractive 'pearmain' shape. This is a fairly dry apple - which is perhaps not regarded as a desirable attribute today. In spite of this it is actually a very enjoyable apple, with a rich aromatic flavour which in apple terms is usually described as Although it had 'shelf appeal' for the Victorian housewife, its autumnal colouring is probably too subdued to compete with the bright young things of the modern supermarket shelves. Perhaps this is part of its appeal; it recalls a bygone era where subtlety of flavour was appreciated - a lovely apple to savour in front of an open fire on a cold winter's day. Tree hardy. Does will in all soils, even clay. AERLIE RED FLESH (Hidden Rose, Mountain Rose) California 1930’s 19 20 20 Cook Oct 20 15 An amazing red fleshed apple, discovered in Aerlie, Oregon, which may be the best of all red fleshed varieties and indeed would be an outstandingly delicious apple no matter what color the flesh is. A choice seedling, Aerlie Red Flesh has a beautiful yellow skin with pale whitish dots, but it is inside that it excels. Deep rose red flesh, juicy, crisp, hard, sugary and richly flavored, ripening late (October) and keeping throughout the winter. The late Conrad Gemmer, an astute observer of apples with 500 varieties in his collection, rated Hidden Rose an outstanding variety of top quality. -

Apple Varieties in Maine Frederick Charles Bradford
The University of Maine DigitalCommons@UMaine Electronic Theses and Dissertations Fogler Library 6-1911 Apple Varieties in Maine Frederick Charles Bradford Follow this and additional works at: http://digitalcommons.library.umaine.edu/etd Part of the Agriculture Commons Recommended Citation Bradford, Frederick Charles, "Apple Varieties in Maine" (1911). Electronic Theses and Dissertations. 2384. http://digitalcommons.library.umaine.edu/etd/2384 This Open-Access Thesis is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of DigitalCommons@UMaine. A thesis submitted to the faculty of the University of Maine in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE IN AGRICULTURE by FREDERICK CHARLES BRADFORD, B. S . Orono, Maine. June, 1911. 8 2 8 5 INTRODUCTION The following pages represent an effort to trace the causes of the changing procession of varieties of apples grown in Maine. To this end the history of fruit growing in Maine has been carefully studied, largely through the Agricultural Reports from 1850 to 1909 and the columns of the Maine Farmer fran 1838 to 1875. The inquiry has been confined as rigidly as possible to this state, out side sources being referred to only for sake of compari son. Rather incidentally, soil influences, modifications due to climate, etc., have been considered. Naturally* since the inquiry was limited to printed record, nothing new has been discovered in this study. Perhaps a somewhat new point of view has been achieved. And, since early Maine pomological literature has been rather neglected by our leading writers, some few forgot ten facts have been exhumed. -

Crop Profile for Apples in Kentucky
CROP PROFILE FOR APPLES IN KENTUCKY SOURCE Title Crop Profile for Apples in Kentucky PDF Document https://ipmdata.ipmcenters.org/documents/cropprofiles/KY_Apple_CropProfile.pdf Type Crop Profile Source Date 04/18/2017 Settings Apple Region Southern States Kentucky Contacts Nicole Gauthier, University of Kentucky, [email protected], (859) 218-0720 Contributors Nicole Gauthier, University of Kentucky CROPS/SETTINGS BACKGROUND Obsolescence of existing documents. Pest occurrences and pest management practices (especially pesticides) have changed dramatically in the last several years. Thus, existing IPM crop profile documents do not resemble today’s orchards or their pest management practices. As mentioned above, there are sixteen apple crop profiles available for reference, with publication dates as late as 1997* (California), 1998* (Kentucky), and 1999* (Ohio). The newest profiles on record were released in 2009 (Tennessee) and 2010 (Virginia). Nonetheless, even the most recent profiles are vastly outdated. Disease priorities/prevalence in both the 1998* Kentucky apple profile and those from neighboring states Tennessee (2009) and Virginia (2010) are not the same as they were at time of publication. For example, apple scab is described as the most consistently serious disease of apple in Kentucky. Powdery mildew appears second in the list (unclear if this order is a ranking), but the disease does not reach epidemic proportions here. Phytophthora root and collar rot only affect young trees in poorly planted situations. The more recent profiles from Tennessee and Virginia, as well as a profile from neighboring North Carolina documented Alternaria blotch, Brooks fruit spot, black pox, and/or blister spot as common diseases. According to UK diagnostic laboratory records (2000 to present) and communication with university specialists, these diseases rarely or never occur in Kentucky. -

Fruit, Nut & Grape Varieties for the Contra Costa Home Orchard
ccmg.ucanr.edu February 2020 Fruit, Nut & Grape Varieties for the Contra Costa Home Orchard by Janet Caprile, Contra Costa County Farm Advisor Emeritus NOTES: The County has been divided into 4 climate zones based on those outlined in the Sunset Western Garden Book. The zones include: Zone 17: Coastal strips Kensington San Pablo Rodeo (bayside) El Cerrito Pinole (bayside) Crockett Richmond Hercules (bayside) Zone 16: Northern California coast thermal belts Orinda (far west) Zone 15: Chilly winters areas along the Coast Range Orinda (central) Martinez (central & west) Walnut Creek (most) El Sobrante Pacheco Alamo (east of Hwy 680) Pinole (inland) Pleasant Hill Danville ( most) Hercules (inland) Concord (most) Rodeo (inland) Clayton Zone 14: Northern California’s inland area with some ocean influence Pittsburg Orinda (east) Alamo (west of Hwy 680) Antioch Moraga Danville (part) Oakley Lafayette Blackhawk Brentwood Walnut Creek (west of Hwy 680) San Ramon Discovery Bay Concord (part) Byron Martinez ( east) Refer to this Sunset website to find your “zone”: https://www.sunset.com/garden/climate-zones/sunset-climate-zone- bay-area LEGEND: COMMONLY GROWN AND COMMONLY AVAILABLE VARIETIES SHOWN IN BOLDFACE TYPE. Parentheses indicate zones that may support the listed fruit variety but are not ideal. v-2020-02-27 1 of 18 The University of California prohibits discrimination or harassment of any person in any of its programs or activities. See the complete Nondiscrimination Statement at ucanr.edu. ccmg.ucanr.edu Fruit, Nut & Grape Varieties for the Contra Costa Home Orchard February 2020 ALMOND Almonds have a low chill requirement (200-300 hours) but need summer heat to mature a crop. -

Handling of Apple Transport Techniques and Efficiency Vibration, Damage and Bruising Texture, Firmness and Quality
Centre of Excellence AGROPHYSICS for Applied Physics in Sustainable Agriculture Handling of Apple transport techniques and efficiency vibration, damage and bruising texture, firmness and quality Bohdan Dobrzañski, jr. Jacek Rabcewicz Rafa³ Rybczyñski B. Dobrzañski Institute of Agrophysics Polish Academy of Sciences Centre of Excellence AGROPHYSICS for Applied Physics in Sustainable Agriculture Handling of Apple transport techniques and efficiency vibration, damage and bruising texture, firmness and quality Bohdan Dobrzañski, jr. Jacek Rabcewicz Rafa³ Rybczyñski B. Dobrzañski Institute of Agrophysics Polish Academy of Sciences PUBLISHED BY: B. DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES ACTIVITIES OF WP9 IN THE CENTRE OF EXCELLENCE AGROPHYSICS CONTRACT NO: QLAM-2001-00428 CENTRE OF EXCELLENCE FOR APPLIED PHYSICS IN SUSTAINABLE AGRICULTURE WITH THE th ACRONYM AGROPHYSICS IS FOUNDED UNDER 5 EU FRAMEWORK FOR RESEARCH, TECHNOLOGICAL DEVELOPMENT AND DEMONSTRATION ACTIVITIES GENERAL SUPERVISOR OF THE CENTRE: PROF. DR. RYSZARD T. WALCZAK, MEMBER OF POLISH ACADEMY OF SCIENCES PROJECT COORDINATOR: DR. ENG. ANDRZEJ STĘPNIEWSKI WP9: PHYSICAL METHODS OF EVALUATION OF FRUIT AND VEGETABLE QUALITY LEADER OF WP9: PROF. DR. ENG. BOHDAN DOBRZAŃSKI, JR. REVIEWED BY PROF. DR. ENG. JÓZEF KOWALCZUK TRANSLATED (EXCEPT CHAPTERS: 1, 2, 6-9) BY M.SC. TOMASZ BYLICA THE RESULTS OF STUDY PRESENTED IN THE MONOGRAPH ARE SUPPORTED BY: THE STATE COMMITTEE FOR SCIENTIFIC RESEARCH UNDER GRANT NO. 5 P06F 012 19 AND ORDERED PROJECT NO. PBZ-51-02 RESEARCH INSTITUTE OF POMOLOGY AND FLORICULTURE B. DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES ©Copyright by BOHDAN DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES LUBLIN 2006 ISBN 83-89969-55-6 ST 1 EDITION - ISBN 83-89969-55-6 (IN ENGLISH) 180 COPIES, PRINTED SHEETS (16.8) PRINTED ON ACID-FREE PAPER IN POLAND BY: ALF-GRAF, UL. -

Danny the Champion of the World
Roald Dahl Danny the Champion of the World 1 The Filling-station When I was four months old, my mother died suddenly and my father was left to look after me all by himself. This is how I looked at the time. I had no brothers or sisters. So all through my boyhood, from the age of four months onward, there were just the two of us, my father and me. We lived in an old gipsy caravan behind a filling-station. My father owned the filling-station and the caravan and a small field behind, but that was about all he owned in the world. It was a very small filling-station on a small country road surrounded by fields and woody hills. While I was still a baby, my father washed me and fed me and changed my nappies and did all the millions of other things a mother normally does for her child. That is not an easy task for a man, especially when he has to earn his living at the same time by repairing motor-car engines and serving customers with petrol. But my father didn’t seem to mind. I think that all the love he had felt for my mother when she was alive he now lavished upon me. During my early years, I never had a moment’s unhappiness or illness and here I am on my fifth birthday. I was now a scruffy little boy as you can see, with grease and oil all over me, but that was because I spent all day in the workshop helping my father with the cars. -

A G E N D a Dakota County Board of Commissioners
A G E N D A Dakota County Board of Commissioners April 6, 2021 9:00 AM Boardroom, Administration Center, Government Center, Hastings, MN View Live Broadcast https://www.co.dakota.mn.us/Government/BoardMeetings/Pages/default.aspx If you wish to speak to an agenda item or an item not on the agenda, please notify the Clerk to the Board via email at [email protected] Emails must be received by 7:30am Tuesday, April 6, 2021. Instructions on how to participate will be sent to anyone interested. 1. Call To Order And Roll Call 2. Pledge Of Allegiance 3. Audience Anyone wishing to address the Committee on an item not on the agenda or an item on the consent agenda may send comments to [email protected] Verbal comments are limited to five minutes. 4. Approval Of Agenda (Additions/Corrections/Deletions) CONSENT AGENDA 5. County Administration - Approval Of Minutes Of Meeting Held On March 23, 2021 6. County Board/County Administration 6.1 County Administration - Authorization To Amend 2021 County Board/Committee Of The Whole Meeting Schedule 6.2 County Administration - Appointments To Public Art Citizen Advisory Committee 7. Community Services 7.1 Public Health - Authorization To Execute Contracts With Cities Of Burnsville, Eagan And Hastings For Provision Of Emergency Medical Services Personnel For COVID-19 Vaccination Clinics 8. Operations, Management And Budget 8.1 Office Of Risk Management - Authorization To Execute Contract With Minnesota Department Of Public Safety For 2020 Urban Area Security Initiative Homeland Security Grant -1- April 6, 2021 Page 2 9. -

Sager Farms Wins Champion Cider for Second
ORCHARD HILL FARM CLAIMS TOP HONORS AT THE 2019 ILLINOIS SWEET CIDER CONTESTS Ken Johnson, Cider Contest Coordinator University of Illinois Extension Educator The Illinois State Horticulture Society sponsored its 30th annual Illinois and National Sweet Cider Contests and the 17th annual National Hard Cider Contest, held in conjunction with the Illinois Specialty Crops, Agritourism and Organic Conference on January 10th in Springfield, Illinois. Wesley Carithers of Orchard Hill Farm located in Lewistown, IL produced the No.1 overall rated cider at this year’s contest, winning both the National and Illinois contests. Wesley used Jonathon, Golden Delicious, Red Delicious, Jonagold and Cameo apples to make his winning cider. Second Place National Cider went to David Doud of Countyline Orchard in Wabash, IN. Second Place Illinois Cider and Third Place National Cider went to Tom Schwartz of Schwartz Orchards in Centralia, IL. Brian Edwards of Edwards Apple Orchard West in Winnebago, IL won Third Place Illinois Cider. Midwest Cider of Merit 1st Runner-up was awarded to Joe Ringhausen of Joe Ringhausen Orchards in Fieldon, IL. Pat Curran and Patrick Riofredo of Curran’s Orchard in Rockford, IL were awarded the Midwest Cider of Merit 2nd Runner-up and the Midwest Cider of Merit 3rd Runner-up went to Randy Graham of Curtis Orchard in Champaign, IL. Grissom’s Lost Creek Orchard of Greenup, IL claimed the Champion Hard Cider award, for the sixth year in a row and eighth overall. Trevor and Dalton Grissom produced their winning hard cider for this year’s contest with a combination of Golden Delicious, GoldRush, Fuji, and Jonathon apples. -

Apple, Reaktion Books
apple Reaktion’s Botanical series is the first of its kind, integrating horticultural and botanical writing with a broader account of the cultural and social impact of trees, plants and flowers. Already published Apple Marcia Reiss Bamboo Susanne Lucas Cannabis Chris Duvall Geranium Kasia Boddy Grasses Stephen A. Harris Lily Marcia Reiss Oak Peter Young Pine Laura Mason Willow Alison Syme |ew Fred Hageneder APPLE Y Marcia Reiss reaktion books Published by reaktion books ltd 33 Great Sutton Street London ec1v 0dx, uk www.reaktionbooks.co.uk First published 2015 Copyright © Marcia Reiss 2015 All rights reserved No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers Printed and bound in China by 1010 Printing International Ltd A catalogue record for this book is available from the British Library isbn 978 1 78023 340 6 Contents Y Introduction: Backyard Apples 7 one Out of the Wild: An Ode and a Lament 15 two A Rose is a Rose is a Rose . is an Apple 19 three The Search for Sweetness 43 four Cider Chronicles 59 five The American Apple 77 six Apple Adulation 101 seven Good Apples 123 eight Bad Apples 137 nine Misplaced Apples 157 ten The Politics of Pomology 169 eleven Apples Today and Tomorrow 185 Apple Varieties 203 Timeline 230 References 234 Select Bibliography 245 Associations and Websites 246 Acknowledgements 248 Photo Acknowledgements 250 Index 252 Introduction: Backyard Apples Y hree old apple trees, the survivors of an unknown orchard, still grow around my mid-nineteenth-century home in ∏ upstate New York. -
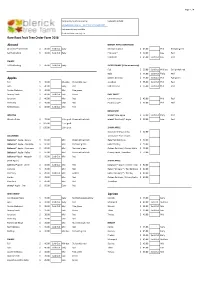
Bare Root Fruit Tree Order Form 2018 Almond Apples Apricot
Page 1 / 4 Place orders by phone or email Updated 14/05/18 [email protected] 0417 535 917 / 5628 1507 Pick up or delivery available Stock available from July 1st Bare Root Fruit Tree Order Form 2018 Almond DWARF APPLE CONTINUED All-in-One™ Self-fertile $ 45.00 Sold Out Early Monty's Surprise $ 45.00 Mid Red and green Self Pollinating $ 40.00 Sold Out Early Pink Lady™ $ 40.00 Late Red Pinkabelle $ 45.00 Sold Out Late Red DWARF Self Pollinating $ 45.00 Sold Out Early SUPER DWARF (1.5m at maturity) Fuji $ 55.00 Sold Out Mid-late Dull pinkish red Gala $ 55.00 Sold Out Early Red Apples Golden Delicious $ 55.00 Sold Out Mid Pale green Fuji $ 40.00 Mid-late Dull pinkish red Jonathan $ 55.00 Sold Out Mid Red Gala $ 40.00 Early Red Red Delicious $ 55.00 Sold Out Mid Red Golden Delicious $ 40.00 Mid Pale green Granny Smith $ 40.00 Sold Out Late Green EASY CARE™ Jonathan $ 40.00 Mid Red Crimson Crisp™ $ 45.00 Mid Red Pink Lady $ 40.00 Late Red Pixie Crunch™ $ 45.00 Mid Red Red Delicious $ 40.00 Sold Out Mid Red MINIATURE WEEPING trixzie® Gala Apple $ 60.00 Sold Out Early Red Wandin Pride $ 70.00 1.2m graft Green with a blush trixzie® Pink Lady™ Apple $ 60.00 Late Red $ 105.00 1.5m graft $ 135.00 1.8m graft 2-WAY APPLE Easy Care Crimson Crisp $ 80.00 COLUMNAR / Easy Care Pixie Crunch Ballerina® Apple - Bolero $ 60.00 Mid Green with a blush Gala/ Red Delicious $ 70.00 Ballerina® Apple - Charlotte $ 60.00 Mid Red over green Gala/ Red Fuji $ 70.00 Ballerina® Apple - Flamenco $ 60.00 Mid Red over green Golden Delicious/ Granny Smith $ 70.00 Ballerina®