Differences in Nectar Traits Between Ornithophilous and Entomophilous Plants on Mount Cameroon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of Extracts of Triclisia Suboardata Oliv and Heinsia Crinita (Afz) G
African Journal of Pharmacy and Pharmacology Vol. 5(2). pp. 125-131, February 2011 Available online http://www.academicjournals.org/ajpp DOI: 10.5897/AJPP10.077 ISSN 1996-0816 ©2011 Academic Journals Full Length Research Paper Evaluation of extracts of Triclisia suboardata Oliv and Heinsia crinita (Afz) G. Taylor for antimicrobial activity against some clinical bacterial isolates and fungi K. A. Abo1*, I. O. Lawal1 and A. Ogunkanmi2 1Department of Pharmacognosy, Faculty of Pharmacy, University of Ibadan, Ibadan, Nigeria. 2Department of Cell Biology and Genetics, Faculty of Science, University of Lagos, Lagos, Nigeria. Accepted 8 January, 2011 We report antimicrobial potential of extracts of roots of Triclisia subcordata and whole plant of Heinsia crinita used as components of various herbal portions in ethnomedine in South West Nigeria to treat acute urinogenital infections and infertility. Methanol and hexane extracts of each plant were obtained by maceration and tested for antimicrobial activity using agar diffusion and microbroth dilution techniques. The extracts were tested against strains of Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa and fungi including four species of Candida. The study showed that extracts of H. crinita and T. subcordata exhibited profound antibacterial activity against the typed and clinical isolates obtained from patients with STD and meningitis. High antifungal activity, particularly against the Candida species was noted as Candida species are implicated in candidiasis and vaginal thrush. Generally, the methanol extract was more effective than the hexane extracts on the test micro-organisms. The study justifies the ethnopharmacological uses of these medicinal plants for treatment of microbial infections. Key words: Triclisia subcardata, Heinsia crinita, antimicrobial activity, non-gonococcal urethritis, infertility. -

Sistema De Clasificación Artificial De Las Magnoliatas Sinántropas De Cuba
Sistema de clasificación artificial de las magnoliatas sinántropas de Cuba. Pedro Pablo Herrera Oliver Tesis doctoral de la Univerisdad de Alicante. Tesi doctoral de la Universitat d'Alacant. 2007 Sistema de clasificación artificial de las magnoliatas sinántropas de Cuba. Pedro Pablo Herrera Oliver PROGRAMA DE DOCTORADO COOPERADO DESARROLLO SOSTENIBLE: MANEJOS FORESTAL Y TURÍSTICO UNIVERSIDAD DE ALICANTE, ESPAÑA UNIVERSIDAD DE PINAR DEL RÍO, CUBA TESIS EN OPCIÓN AL GRADO CIENTÍFICO DE DOCTOR EN CIENCIAS SISTEMA DE CLASIFICACIÓN ARTIFICIAL DE LAS MAGNOLIATAS SINÁNTROPAS DE CUBA Pedro- Pabfc He.r retira Qltver CUBA 2006 Tesis doctoral de la Univerisdad de Alicante. Tesi doctoral de la Universitat d'Alacant. 2007 Sistema de clasificación artificial de las magnoliatas sinántropas de Cuba. Pedro Pablo Herrera Oliver PROGRAMA DE DOCTORADO COOPERADO DESARROLLO SOSTENIBLE: MANEJOS FORESTAL Y TURÍSTICO UNIVERSIDAD DE ALICANTE, ESPAÑA Y UNIVERSIDAD DE PINAR DEL RÍO, CUBA TESIS EN OPCIÓN AL GRADO CIENTÍFICO DE DOCTOR EN CIENCIAS SISTEMA DE CLASIFICACIÓN ARTIFICIAL DE LAS MAGNOLIATAS SINÁNTROPAS DE CUBA ASPIRANTE: Lie. Pedro Pablo Herrera Oliver Investigador Auxiliar Centro Nacional de Biodiversidad Instituto de Ecología y Sistemática Ministerio de Ciencias, Tecnología y Medio Ambiente DIRECTORES: CUBA Dra. Nancy Esther Ricardo Ñapóles Investigador Titular Centro Nacional de Biodiversidad Instituto de Ecología y Sistemática Ministerio de Ciencias, Tecnología y Medio Ambiente ESPAÑA Dr. Andreu Bonet Jornet Piiofesjar Titular Departamento de EGdfegfe Universidad! dte Mearte CUBA 2006 Tesis doctoral de la Univerisdad de Alicante. Tesi doctoral de la Universitat d'Alacant. 2007 Sistema de clasificación artificial de las magnoliatas sinántropas de Cuba. Pedro Pablo Herrera Oliver I. INTRODUCCIÓN 1 II. ANTECEDENTES 6 2.1 Historia de los esquemas de clasificación de las especies sinántropas (1903-2005) 6 2.2 Historia del conocimiento de las plantas sinantrópicas en Cuba 14 III. -

January 2012 ---International Rock Gardener--- January 2012
International Rock Gardener Number 25 The Scottish Rock Garden Club January 2012 ---International Rock Gardener--- January 2012 We begin the year with some “perennial favourites”: plants with lasting attraction. The late Harold Esslemont was one of the most experienced growers and exhibitors in the SRGC over a great many years and the following article was adapted from The Rock Garden journal of 1969 to showcase some plants that are as popular today as they were over forty years ago. The last cover of 2011 was of a wintry scene in the Scottish Garden of two of the IRG team so we thought we’d share this January sunset for the start of 2012. In his weekly Bulb Log Diary, now in its tenth year, Ian shares his method of taking such photos. Cover picture: January Sunset, Aberdeen. J. Ian Young ---Mountains in the Garden--- My Twelve Favourite Alpines by the late Harold Esslemont M.B.E. (adapted by M.Y.) It was the final meeting of the season of the local group. A postcard announced that two* members had been invited to show and discuss slides of their twelve favourite alpines. It appeared that I was to be one of the speakers. I forget who told me that his list of twelve favourite alpines ran to at least twenty, but I was soon to learn how right he was. My brief was twelve plants, no more, and a decision, however difficult, had to be made. The compiling of such a list is influenced by so many factors that the result may be expected to vary widely among individuals. -

In Vitro Culture of Luma Chequen from Vegetative Buds
Cien. Inv. Agr. 40(3):609-615. 2013 www.rcia.uc.cl BIOTECHNOLOGY RESEARCH NOTE In vitro culture of Luma chequen from vegetative buds Héctor Mancilla1, Karla Quiroz1, Ariel Arencibia1, Basilio Carrasco2, and Rolando García-Gonzales1 1Facultad de Ciencias Agrarias y Forestales, Universidad Católica del Maule, Campus San Miguel. Casilla 617, Talca, Chile. 2Facultad de Agronomía, Pontificia Universidad Católica de Chile. Ave. Vicuña Mackenna 4860, Macul, Santiago, Chile. Abstract H. Mancilla, K. Quiroz, A. Arencibia, B. Carrasco, and R. García-Gonzales. 2013. In vitro culture of Luma chequen from vegetative buds. Cien. Inv. Agr. 40(3): 609-615. Luma chequen, a small tree or large shrub belonging to the Myrtaceae family, is endemic to South America and has medicinal, nutritional and ornamental potential. However, its native habitat is deteriorating gradually, and it is suffering from the effects of fragmentation that is being caused by the conversion of forest land to agricultural land and the natural expansion of monocultural plantations of exotic species, such as Pinus radiata. The purpose of this work is to develop an effective procedure for establishing in vitro cultures of the native Chilean species L. chequen. Aseptic nodal segments were evaluated after exposure to a disinfecting agent (1% solution of sodium hypochlorite) for different lengths of time. Murashige and Skoog (MS) or Woody Plant (WPM) culture media with 6-Bencilaminopurine (BAP) or 2-isopentenil adenine (2-iP) added to a concentration of 1 mg L-1 were evaluated. Although no significant differences were observed between cultures with and without additives, 40.43% of the explant cultures were successfully established. -

Antiplasmodial Activities and in Vivo Safety of Extracts and Compounds of Seven Indigenous Kenyan Medicinal Plants Used Traditionally to Treat Malaria
i ANTIPLASMODIAL ACTIVITIES AND IN VIVO SAFETY OF EXTRACTS AND COMPOUNDS OF SEVEN INDIGENOUS KENYAN MEDICINAL PLANTS USED TRADITIONALLY TO TREAT MALARIA ONYOYO SAMWEL GUYA I56/8873/99 A RESEARCH THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE AWARD OF THE DEGREE OF MASTER OF SCIENCE IN BIOCHEMISTRY, KENYATTA UNIVERSITY OCTOBER, 2020 ii DECLARATION AND DEDICATION I declare that this thesis is my original work and it has not been presented for a degree in any other University or for any other award. I dedicate this work to my late father mzee Festus Onyoyo Ouya who was the source of my aspiration as he had a special aspiration towards acquiring knowledge. Onyoyo Samwel Guya Reg/No: 156/8873/99 Signature______________________ Date_________________ We confirm that the work reported in this thesis was carried out by the candidate under our supervision Supervisors Prof Eliud NM Njagi Department of Biochemistry, Microbiology and Biotechnology Kenyatta University P.O Box 43844, Nairobi Signature______________________ Date_________________ Prof Anastasia N Guantai Department of Pharmacology and Pharmacognosy University of Nairobi P.O Box 19676, Nairobi Signature______________________ Date__________________ Prof Caroline C Langat-Thoruwa Department of Chemistry Kenyatta University P.O Box 43844, Nairobi Signature_____________________ Date___________________ iii TABLE OF CONTENTS DECLARATION AND DEDICATION .................................................................. ii TABLE OF CONTENTS ........................................................................................ -

Supplementary Material
Supplementary material Initial responses in growth, production, and regeneration following selection cuttings with varying residual densities in hardwood-dominated temperate rainforests in Chile Height and diameter functions, adjusted following the Stage’s model ([35]; equation S1). µ h=1,3+ α ∗(∗ ) [S1] Where: α, β, μ: parameters to be estimated; dbh: diameter at breast height (cm); h = total height (m). Table S1 Parameters and measures of goodness of fit and prediction of height-diameter functions in Llancahue (LL). n: number of samples. Parameter DA RMSE R2 Species n α β µ (%) (%) (%) Aextoxicon punctatum 69.33 5.35 0.41 0.08 14.42 87 30 Drimys winteri 32.04 4.46 0.59 -0.70 9.42 92 30 Eucryphia cordifolia 58.08 4.13 0.41 0.99 12.00 85 57 Laureliopsis philippiana 56.20 5.30 0.47 0.48 13.57 78 78 Long-lived intolerant 49.62 3.46 0.38 -0.08 14.58 72 16 Myrtaceae 147.06 4.81 0.25 1.48 16.87 75 30 Other species 44.48 4.61 0.43 0.53 17.92 70 31 Podocarpaceae 61.13 5.01 0.40 0.18 13.57 89 26 Proteaceae 31.32 2.82 0.43 -1.25 16.61 50 22 Notes: Long-lived intolerant: Nothofagus dombeyi, Weinmannia trichosperma; Myrtaceae: Amomyrtus luma, Amomyrtus meli, Luma apiculate;Podocarpaceae: Podocarpus salignus, Podocarpus nubigenus, Saxegothaea conspicua; Proteaceae: Gevuina avellana, Lomatia ferruginea, Lomatia dentata. DA and RMSE are measures of goodness of prediction: DA (Aggregated difference), RMSE (Root mean square error). -
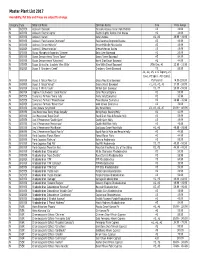
Master Plant List 2017.Xlsx
Master Plant List 2017 Availability, Pot Size and Prices are subject to change. Category Type Botanical Name Common Name Size Price Range N BREVER Azalea X 'Cascade' Cascade Azalea (Glenn Dale Hybrid) #3 49.99 N BREVER Azalea X 'Electric Lights' Electric Lights Double Pink Azalea #2 44.99 N BREVER Azalea X 'Karen' Karen Azalea #2, #3 39.99 - 49.99 N BREVER Azalea X 'Poukhanense Improved' Poukhanense Improved Azalea #3 49.99 N BREVER Azalea X 'Renee Michelle' Renee Michelle Pink Azalea #3 49.99 N BREVER Azalea X 'Stewartstonian' Stewartstonian Azalea #3 49.99 N BREVER Buxus Microphylla Japonica "Gregem' Baby Gem Boxwood #2 29.99 N BREVER Buxus Sempervirens 'Green Tower' Green Tower Boxwood #5 64.99 N BREVER Buxus Sempervirens 'Katerberg' North Star Dwarf Boxwood #2 44.99 N BREVER Buxus Sinica Var. Insularis 'Wee Willie' Wee Willie Dwarf Boxwood Little One, #1 13.99 - 21.99 N BREVER Buxus X 'Cranberry Creek' Cranberry Creek Boxwood #3 89.99 #1, #2, #5, #15 Topiary, #5 Cone, #5 Spiral, #10 Spiral, N BREVER Buxus X 'Green Mountain' Green Mountain Boxwood #5 Pyramid 14.99-299.99 N BREVER Buxus X 'Green Velvet' Green Velvet Boxwood #1, #2, #3, #5 17.99 - 59.99 N BREVER Buxus X 'Winter Gem' Winter Gem Boxwood #5, #7 59.99 - 99.99 N BREVER Daphne X Burkwoodii 'Carol Mackie' Carol Mackie Daphne #2 59.99 N BREVER Euonymus Fortunei 'Ivory Jade' Ivory Jade Euonymus #2 35.99 N BREVER Euonymus Fortunei 'Moonshadow' Moonshadow Euonymus #2 29.99 - 35.99 N BREVER Euonymus Fortunei 'Rosemrtwo' Gold Splash Euonymus #2 39.99 N BREVER Ilex Crenata 'Sky Pencil' -

South Cameroon)
Plant Ecology and Evolution 152 (1): 8–29, 2019 https://doi.org/10.5091/plecevo.2019.1547 CHECKLIST Mine versus Wild: a plant conservation checklist of the rich Iron-Ore Ngovayang Massif Area (South Cameroon) Vincent Droissart1,2,3,8,*, Olivier Lachenaud3,4, Gilles Dauby1,5, Steven Dessein4, Gyslène Kamdem6, Charlemagne Nguembou K.6, Murielle Simo-Droissart6, Tariq Stévart2,3,4, Hermann Taedoumg6,7 & Bonaventure Sonké2,3,6,8 1AMAP Lab, IRD, CIRAD, CNRS, INRA, Université de Montpellier, Montpellier, France 2Missouri Botanical Garden, Africa and Madagascar Department, P.O. Box 299, St. Louis, Missouri 63166-0299, U.S.A. 3Herbarium et Bibliothèque de Botanique africaine, C.P. 265, Université Libre de Bruxelles, Campus de la Plaine, Boulevard du Triomphe, BE-1050 Brussels, Belgium 4Meise Botanic Garden, Domein van Bouchout, Nieuwelaan 38, BE-1860 Meise, Belgium 5Evolutionary Biology and Ecology, Faculté des Sciences, C.P. 160/12, Université Libre de Bruxelles, 50 Avenue F. Roosevelt, BE-1050 Brussels, Belgium 6Plant Systematics and Ecology Laboratory, Higher Teachers’ Training College, University of Yaoundé I, P.O. Box 047, Yaoundé, Cameroon 7Bioversity International, P.O. Box 2008 Messa, Yaoundé, Cameroon 8International Joint Laboratory DYCOFAC, IRD-UYI-IRGM, BP1857, Yaoundé, Cameroon *Author for correspondence: [email protected] Background and aims – The rapid expansion of human activities in South Cameroon, particularly mining in mountainous areas, threatens this region’s exceptional biodiversity. To comprehend the effects of land- use change on plant diversity and identify conservation priorities, we aim at providing a first comprehensive plant checklist of the Ngovayang Massif, focusing on the two richest plant families, Orchidaceae and Rubiaceae. -

Kew Science Publications for the Academic Year 2017–18
KEW SCIENCE PUBLICATIONS FOR THE ACADEMIC YEAR 2017–18 FOR THE ACADEMIC Kew Science Publications kew.org For the academic year 2017–18 ¥ Z i 9E ' ' . -,i,c-"'.'f'l] Foreword Kew’s mission is to be a global resource in We present these publications under the four plant and fungal knowledge. Kew currently has key questions set out in Kew’s Science Strategy over 300 scientists undertaking collection- 2015–2020: based research and collaborating with more than 400 organisations in over 100 countries What plants and fungi occur to deliver this mission. The knowledge obtained 1 on Earth and how is this from this research is disseminated in a number diversity distributed? p2 of different ways from annual reports (e.g. stateoftheworldsplants.org) and web-based What drivers and processes portals (e.g. plantsoftheworldonline.org) to 2 underpin global plant and academic papers. fungal diversity? p32 In the academic year 2017-2018, Kew scientists, in collaboration with numerous What plant and fungal diversity is national and international research partners, 3 under threat and what needs to be published 358 papers in international peer conserved to provide resilience reviewed journals and books. Here we bring to global change? p54 together the abstracts of some of these papers. Due to space constraints we have Which plants and fungi contribute to included only those which are led by a Kew 4 important ecosystem services, scientist; a full list of publications, however, can sustainable livelihoods and natural be found at kew.org/publications capital and how do we manage them? p72 * Indicates Kew staff or research associate authors. -

RHS the Garden Index 2011: Volume 136, Parts 1-12
January 2011 I £4.25 I www.rhs.org.uk February 2011 I £4.25 I www.rhs.org.uk March 2011 I £4.25 I www.rhs.org.uk April 2011 I £4.25 I www.rhs.org.uk This month: June 2011 I £4.25 I www.rhs.org.uk July 2011 I £4.25 I www.rhs.org.uk WINTER GARDEN DELIGHT VINTAGE DAFFODILS IDEAS FOR STAKING PERENNIALS GUIDE TO USING PEAT-FREE COMPOST The December 2011 | www.rhs.org.uk | £4.25 August 2011 I £4.25 I www.rhs.org.uk Garden VIBURNUMS FOR WINTER ANNUALS WITHA POTTED PASSION FOR APPE AL HOUSE PLANTS How to grow moth orchidsCHELSEA CHAMPIONS PRODUCTIVE CONTAINERS ASTRANTIAS FOR SUMMER STYLE FLOWERING CHERRIES OLD ANDPOPPIES NEW ON THE MARCHquinces: SWEET DIVERSITY IN HONEYSUCKLEScelebrating a forgotten fruit VARIEGATED EVERGREENS Index 2011: Volume 136, Parts 1–12 INDULGE IN GINGERS CURIOUS CUCUMBER RELATIVES Aug11 Cover_August 2011_The Garden_ 1 14/07/2011 11:28 Fruiting shrubs to attract birds Index 2011 January 2011 I £4.25 I www.rhs.org.uk February 2011 I £4.25 I www.rhs.org.uk March 2011 I £4.25 I www.rhs.org.uk April 2011 I £4.25 I www.rhs.org.uk May 2011 I £4.25 I www.rhs.org.uk June 2011 I £4.25 I www.rhs.org.uk This month: WINTER GARDEN DELIGHT VIBURNUMS FOR WINTER VINTAGE DAFFODILS PRODUCTIVE CONTAINERS BORDER BRILLIANCE POPPIES ON THE MARCH GUIDE TO USING PEAT-FREE COMPOST ANNUALS WITH POTTED APPEAL IDEAS FOR STAKING PERENNIALS FLOWERING CHERRIES OLD AND NEW DISPELLING WISTERIA MYTHS SWEET DIVERSITY IN HONEYSUCKLES January 2011 February 2011 March 2011 April 2011 May 2011 June 2011 1 pp1–68 2 pp69–142 3 pp143–214 4 pp215–286 5 pp287–364 6 pp365–432 Numbers in bold before Frutti Series) 6: 407, caterpillar 8: 529 vineale 5: 349 the page number(s) 407 readers’ response allotments: AWARD OF GARDEN MERIT denote the part number Achimenes: 10: 17 keeping them (month). -

The Relationship Between Ecosystem Services and Urban Phytodiversity Is Be- G.M
Open Journal of Ecology, 2020, 10, 788-821 https://www.scirp.org/journal/oje ISSN Online: 2162-1993 ISSN Print: 2162-1985 Relationship between Urban Floristic Diversity and Ecosystem Services in the Moukonzi-Ngouaka Neighbourhood in Brazzaville, Congo Victor Kimpouni1,2* , Josérald Chaîph Mamboueni2, Ghislain Bileri-Bakala2, Charmes Maïdet Massamba-Makanda2, Guy Médard Koussibila-Dibansa1, Denis Makaya1 1École Normale Supérieure, Université Marien Ngouabi, Brazzaville, Congo 2Institut National de Recherche Forestière, Brazzaville, Congo How to cite this paper: Kimpouni, V., Abstract Mamboueni, J.C., Bileri-Bakala, G., Mas- samba-Makanda, C.M., Koussibila-Dibansa, The relationship between ecosystem services and urban phytodiversity is be- G.M. and Makaya, D. (2020) Relationship ing studied in the Moukonzi-Ngouaka district of Brazzaville. Urban forestry, between Urban Floristic Diversity and Eco- a source of well-being for the inhabitants, is associated with socio-cultural system Services in the Moukonzi-Ngouaka Neighbourhood in Brazzaville, Congo. Open foundations. The surveys concern flora, ethnobotany, socio-economics and Journal of Ecology, 10, 788-821. personal interviews. The 60.30% naturalized flora is heterogeneous and https://doi.org/10.4236/oje.2020.1012049 closely correlated with traditional knowledge. The Guineo-Congolese en- demic element groups are 39.27% of the taxa, of which 3.27% are native to Received: September 16, 2020 Accepted: December 7, 2020 Brazzaville. Ethnobotany recognizes 48.36% ornamental taxa; 28.36% food Published: December 10, 2020 taxa; and 35.27% medicinal taxa. Some multiple-use plants are involved in more than one field. The supply service, a food and phytotherapeutic source, Copyright © 2020 by author(s) and provides the vegetative and generative organs. -

A New Species in the Tree Genus Polyceratocarpus (Annonaceae) from the Udzungwa Mountains of Tanzania Andrew Marshall, Thomas L.P
A new species in the tree genus Polyceratocarpus (Annonaceae) from the Udzungwa Mountains of Tanzania Andrew Marshall, Thomas L.P. Couvreur, Abigail Summers, Nicolas Deere, W.R. Quentin Luke, Henry Ndangalasi, Sue Sparrow, David Johnson To cite this version: Andrew Marshall, Thomas L.P. Couvreur, Abigail Summers, Nicolas Deere, W.R. Quentin Luke, et al.. A new species in the tree genus Polyceratocarpus (Annonaceae) from the Udzungwa Mountains of Tanzania. PhytoKeys, Pensoft, 2016, 63, pp.63-76. 10.3897/phytokeys.63.6262. hal-03275053 HAL Id: hal-03275053 https://hal.archives-ouvertes.fr/hal-03275053 Submitted on 30 Jun 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License A peer-reviewed open-access journal PhytoKeys 63: 63–76 (2016)A new species in the tree genus Polyceratocarpus (Annonaceae)... 63 doi: 10.3897/phytokeys.63.6262 RESEARCH ARTICLE http://phytokeys.pensoft.net Launched to accelerate biodiversity research A new species in the tree genus Polyceratocarpus (Annonaceae) from the Udzungwa Mountains of Tanzania Andrew R. Marshall1,2, Th omas L.P. Couvreur4,5,6, Abigail L. Summers1,2,3, Nicolas J.