Conyza Bonariensis Glyphosate Tolerance, As Affected by Origin, Temperature and Growth Stage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sea Level Rise and Flood Risk Assessment for a Select Disaster Prone Area Along the Western Cape Coast
Department of Environmental Affairs and Development Planning Sea Level Rise and Flood Risk Assessment for a Select Disaster Prone Area Along the Western Cape Coast Phase 2 Report: Eden District Municipality Sea Level Rise and Flood Risk Modelling Final May 2010 REPORT TITLE : Phase 2 Report: Eden District Municipality Sea Level Rise and Flood Risk Modelling CLIENT : Provincial Government of the Western Cape Department of Environmental Affairs and Development Planning: Strategic Environmental Management PROJECT : Sea Level Rise and Flood Risk Assessment for a Select Disaster Prone Area Along the Western Cape Coast AUTHORS : D. Blake N. Chimboza REPORT STATUS : Final REPORT NUMBER : 769/2/1/2010 DATE : May 2010 APPROVED FOR : S. Imrie D. Blake Project Manager Task Leader This report is to be referred to in bibliographies as: Umvoto Africa. (2010). Sea Level Rise and Flood Risk Assessment for a Select Disaster Prone Area Along the Western Cape Coast. Phase 2 Report: Eden District Municipality Sea Level Rise and Flood Risk Modelling. Prepared by Umvoto Africa (Pty) Ltd for the Provincial Government of the Western Cape Department of Environmental Affairs and Development Planning: Strategic Environmental Management (May 2010). Phase 2: Eden DM Sea Level Rise and Flood Risk Modelling 2010 EXECUTIVE SUMMARY INTRODUCTION Umvoto Africa (Pty) Ltd was appointed by the Western Cape Department of Environmental Affairs and Development Planning (DEA&DP): Strategic Environmental Management division to undertake a sea level rise and flood risk assessment for a select disaster prone area along the Western Cape coast, namely the portion of coastline covered by the Eden District (DM) Municipality, from Witsand to Nature’s Valley. -

Provincial Gazette Extraordinary Buitengewone Provinsiale Koerant
PROVINCE OF WESTERN CAPE PROVINSIE WES-KAAP Provincial Gazette Buitengewone Extraordinary Provinsiale Koerant 6930 6930 Friday, 23 December 2011 Vrydag, 23 Desember 2011 Registered at the Post Offıce as a Newspaper CONTENTS (*Reprints are obtainable at Room M21, Provincial Legislature Building, 7 Wale Street, Cape Town 8001.) No. Page Provincial Notice 333 Consolidated Annual Municipal Performance Report: 2009/10 .............................................................................. 2 2 Province of the Western Cape: Provincial Gazette Extraordinary 6930 23 December 2011 PROVINCIAL NOTICE PROVINSIALE KENNISGEWING The following Provincial Notice is published for general informa- Die volgende Provinsiale Kennisgewing word vir algemene inligting tion. gepubliseer. ADV. B. GERBER, ADV. B. GERBER, DIRECTOR-GENERAL DIREKTEUR-GENERAAL Provincial Building, Provinsiale-gebou, Wale Street, Waalstraat, Cape Town. Kaapstad. P.N. 333/2011 23 December 2011 Provincial Notice CONSOLIDATED ANNUAL MUNICIPAL PERFORMANCE REPORT 2009/10 23 Desember 2011 Provinsie Wes-Kaap: Buitengewone Provinsiale Koerant 6930 3 CONTENTS FOREWORD BY MEC vi LIST OF TABLES viii EXECUTIVE SUMMARY x LEGISLATIVE OVERVIEW xiii RESEARCH METHODOLOGY xvi CHAPTER 1: GEOGRAPHIC, DEMOGRAPHIC AND SOCIO- ECONOMIC PROFILES OF MUNICIPALITIES 1.1 Introduction 1 1.2 Geographic information 1 1.3 Demographic information 3 1.4 Socio-economic information 4 1.5 Political composition 5 1.6 Classification of municipal capacity by the National Treasury 6 1.7 Assessment of demographic and socio-economic -

Regional Development Profile: Eden District 2010 Working Paper
Provincial Government Western Cape Provincial Treasury Regional Development Profile: Eden District 2010 Working paper To obtain additional information of this document, please contact: Western Cape Provincial Treasury Directorate Budget Management: Local Government Private Bag X9165 7 Wale Street Cape Town Tel: (021) 483-3386 Fax: (021) 483-4680 This publication is available online at http://www.capegateway.gov.za Contents Chapter 1: Eden District Municipality Introduction 3 1. Demographics 4 2. Socio-economic Development 8 3. Labour 18 4. Economy 23 5. Built Environment 26 6. Finance and Resource Mobilisation 37 7. Political Composition 41 8. Environmental Management 41 Cautionary Note 47 Chapter 2: Kannaland Local Municipality Introduction 51 1. Demographics 52 2. Socio-economic Development 55 3. Labour 63 4. Economy 68 5. Built Environment 70 6. Finance and Resource Mobilisation 74 7. Governance and Institutional Development 77 Cautionary Note 78 Chapter 3: Hessequa Local Municipality Introduction 83 1. Demographics 84 2. Socio-development 87 3. Labour 95 4. Economy 100 5. Built Environment 102 6. Finance and Resource Mobilisation 107 7. Political Composition 110 Cautionary Note 111 i REGIONAL DEVELOPMENT PROFILE 2010 Chapter 4: Mossel Bay Local Municipality Introduction 115 1. Demographics 116 2. Socio-development 120 3. Labour 130 4. Economy 135 5. Built Environment 137 6. Finance and Resource Mobilisation 141 7. Political Composition 145 8. Environmental Management 145 Cautionary Note 149 Chapter 5: George Local Municipality Introduction 153 1. Demographics 154 2. Socio-economic Development 158 3. Labour 167 4. Economy 172 5. Built environment 174 6. Finance and Resource Mobilisation 179 7. Political Composition 182 Cautionary Note 183 Chapter 6: Oudtshoorn Local Municipality Introduction 187 1. -

Directory of Organisations and Resources for People with Disabilities in South Africa
DISABILITY ALL SORTS A DIRECTORY OF ORGANISATIONS AND RESOURCES FOR PEOPLE WITH DISABILITIES IN SOUTH AFRICA University of South Africa CONTENTS FOREWORD ADVOCACY — ALL DISABILITIES ADVOCACY — DISABILITY-SPECIFIC ACCOMMODATION (SUGGESTIONS FOR WORK AND EDUCATION) AIRLINES THAT ACCOMMODATE WHEELCHAIRS ARTS ASSISTANCE AND THERAPY DOGS ASSISTIVE DEVICES FOR HIRE ASSISTIVE DEVICES FOR PURCHASE ASSISTIVE DEVICES — MAIL ORDER ASSISTIVE DEVICES — REPAIRS ASSISTIVE DEVICES — RESOURCE AND INFORMATION CENTRE BACK SUPPORT BOOKS, DISABILITY GUIDES AND INFORMATION RESOURCES BRAILLE AND AUDIO PRODUCTION BREATHING SUPPORT BUILDING OF RAMPS BURSARIES CAREGIVERS AND NURSES CAREGIVERS AND NURSES — EASTERN CAPE CAREGIVERS AND NURSES — FREE STATE CAREGIVERS AND NURSES — GAUTENG CAREGIVERS AND NURSES — KWAZULU-NATAL CAREGIVERS AND NURSES — LIMPOPO CAREGIVERS AND NURSES — MPUMALANGA CAREGIVERS AND NURSES — NORTHERN CAPE CAREGIVERS AND NURSES — NORTH WEST CAREGIVERS AND NURSES — WESTERN CAPE CHARITY/GIFT SHOPS COMMUNITY SERVICE ORGANISATIONS COMPENSATION FOR WORKPLACE INJURIES COMPLEMENTARY THERAPIES CONVERSION OF VEHICLES COUNSELLING CRÈCHES DAY CARE CENTRES — EASTERN CAPE DAY CARE CENTRES — FREE STATE 1 DAY CARE CENTRES — GAUTENG DAY CARE CENTRES — KWAZULU-NATAL DAY CARE CENTRES — LIMPOPO DAY CARE CENTRES — MPUMALANGA DAY CARE CENTRES — WESTERN CAPE DISABILITY EQUITY CONSULTANTS DISABILITY MAGAZINES AND NEWSLETTERS DISABILITY MANAGEMENT DISABILITY SENSITISATION PROJECTS DISABILITY STUDIES DRIVING SCHOOLS E-LEARNING END-OF-LIFE DETERMINATION ENTREPRENEURIAL -

05 Agosto Collegamento Diretto 380Km / 4H 50’
Port Elizabeth - Mossel Bay!Km 415,2 05 Agosto Collegamento diretto 380Km / 4h 50’ Port Elizabeth - Robberg Nature Reserve West Cape - 251Km - 3h 15ʼ Partenza : 06.00 Arrivo : 09.15 Tempo di visita : circa 3 ore Robberg Nature Reserve Visita della Riserva naturale della Penisola di Robberg, possibilità di scegliere tra 4 sentieri da decidere direttamente sul posto (vedi mappa dei sentieri allegata) Robberg Nature Reserve - Knysna West Cape - 40,8 Km - 37ʼ Partenza : 12.15 Arrivo : 12.55 Tempo di visita : circa 40ʼ Knysna Sosta dedicata al rifornimento auto, pranzo in Main Road, la via commerciale con il maggior numero di locali per pasti anche veloci. Knysna - Goukamma Nature Reserve West Cape - 19,4 Km - 22ʼ Partenza : 13.40 Arrivo : 14.00 Tempo di visita : circa 2 ore e 30ʼ Goukamma Nature Reserve Visita dell’area marina protetta. Poco prima di Buffalo Bay si trovano gli uffici e l’ingres- so al parco. Possibilità di percorrere alcuni sentieri di varia lunghezza, vedi pdf allega- to. Il parco chiude alle 18.00 Goukamma Nature Reserve - Mossel Bay West Cape - 104 Km - 1h 30ʼ Partenza : 16.30 Arrivo : 18.00 Tempo di visita : cena e pernottamento Park House Lodge 121 High Street Mossel Bay 6500 - +27 044 691 1937 [email protected] Port Elizabeth - Robberg Nature Reserve West Cape - 251Km - 3h 15ʼ Port Elizabeth - Robberg Nature Reserve - DETTAGLI West Cape - 251Km - 3h 15ʼ da Chapman Hotel & Conference Centre a Robberg Nature R... http://maps.google.it/maps?f=d&source=s_d&saddr=Brooke... Indicazioni stradali per Robberg Nature Reserve Plettenberg bay, West Cape, South Africa 251 km – circa 3 ore 15 min 05 AGOSTO Chapman Hotel & Conference Centre Brookes Hill Drive, Port Elizabeth 6001, South Africa - 041 584 0678 1. -

Western Cape Association for Play Therapy Wes-Kaap Vereniging Vir Spelterapie Application Form
WESTERN CAPE ASSOCIATION FOR PLAY THERAPY WES-KAAP VERENIGING VIR SPELTERAPIE APPLICATION FORM DAY VISITOR ANNUAL MEMBERSHIP Name Surname Work number Cell phone number Work email Personal email Occupation Organisation Area(s) of service rendering Please turn the page and circle your answers If registered, which council? Registration number Do you work privately? [Mark with an X] Yes No If yes, please specify the type of service Do you provide play therapy? Yes No Membership fees paid by Self Employer Date Signature Can we add your info to the external resource database? Yes No FOR OFFICE USE ONLY Payment EFT Cash Invoice number Membership number Please be advised that all information provided should be updated with WCA for Play Therapy in the event of change CENTRAL KAROO Beaufort West Laingsburg Leeu-Gamka Matjiesfontein Merweville Murraysburg Nelspoort Prince Albert CAPE WINELANDS Ashton Bonnievale Ceres De Doorns Denneburg Franschhoek Gouda Kayamandi Klapmuts Kylemore Languedoc McGregor Montagu Op-die-Berg Paarl Pniel Prince Alfred Hamlet Rawsonville Robertson Robertsvlei Rozendal Saron Stellenbosch Touws River Tulbagh Wellington Wemmershoek Wolseley Worcester CAPE METROPOLE Atlantis Bellville Blue Downs Brackefell Cape Town Crossroads Durbanville Eerste River Elsie's River Fish Hoek Goodwood Gordon's Bay Gugulethu Hout Bay Khayelitsha Kraaifontein Kuils River Langa Macassar Melkbosstrand Mfuleni Milnerton Mitchell's Plain Noordhoek Nyanga Observatory Parow Simon's Town Somerset West Southern Suburbs Strand EDEN Albertinia Boggomsbaai -
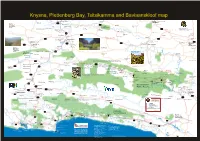
Knysna, Plettenberg Bay, Tsitsikamma and Baviaanskloof Map 2020.Cdr
Knysna, Plettenberg Bay, TsitsikammaMILLER and Baviaanskloof map Van Rooyenskraal Guest Farm Road to Graaf Reinet Koedoeskloof Road to Road to Jansenville River Blydefontein Graaf Reinet Tented Camp R306 Naroegaspoort Oppi-Vlak Farm Stall & Road to: MOUNT STEWART Karoo Farm Cottage R337 Prins Albert Toornberg Beerpoort 173Km Trompetterspoort Kamferspoort Gamkaskloof Angora Mohair Timbila (Willowmore to Graaf Reinet) Pass Die Hel Pass Cottages Game Reserve Grootrivier Blydefontein Tented Camp Glenmore R75 Copy Right © 2020 Gerhard Brummer Witteberge Farm Cottage Bhejane 209Km +27 (0)72 664 3937 www.krugertocape.co.za Perdepoort Pass (Graaf Reinet to Port Elizabeth) No part of this map shall be copied in any way or means Game Reserve without the permission of the copy right holder Kumnandi Lodge Marlu Guest Farm Die Wonderboom Pienaarspoort BAROE N9 Driekuilen Medenpoort Guest Farm R329 Pass Aasvoëlberg Waaipoort Salt Pan Nek Sandkraalpoort Pass Airport 90Km Noorspoort Seekoeinek Soetendalspoort (Willowmore to Steytlerville) Grootrivier Guest Farm R407 R329 WOLWEFONTEIN Finchley Farm Cottage WILLOWMORE Wolwefontein B&B / SC / Camping Hotel Royal Hotel Strydomsvlei Willowmore Hospital The Old Jail Guest House Karoo Theatrical Hotel * Self catering Accommodation R75 Laughing & Art Gallery * Sheep Farming Noorspoort Haaspoort Bush Camp Road to Waters Dam R329 Steyterville Villa & Nature Reserve R332 Kirkwood Vondeling Opel Crafts Zandibela Royal Hotel Steyterville R407 [Angel Factory] Private Game Lodge STEYTLERVILLE Addo * Accommodation Port -

The Garden Route Golfscape: a Golfing Destination in the Rough
THE GARDEN ROUTE GOLFSCAPE: A GOLFING DESTINATION IN THE ROUGH LOUISE-MARI VAN ZYL Thesis presented in partial fulfilment of the requirements for the degree of Master of Arts at the University of Stellenbosch. Supervisor: Mr. BH Schloms December 2006 ii DECLARATION I, the undersigned, hereby declare that the work contained in this thesis is my own original work and that I have not previously, in its entirety or in part, submitted it at any university for a degree. Signature: Date: iii SUMMARY The Garden Route is located along the southern Cape coast of South Africa, between the Outeniqua Mountain Range and the coast, stretching from Gourits River in the west to Bloukranz River in the east. This region is recognised as a holiday destination and the centre of the southern Cape’s tourism industry. It has also gained popularity as a golfing destination set to proliferate in terms of new golf- course developments (Golf Digest 2004; Gould 2004; Granger 2003). No known complete academic or public record is however available for the study area in which all the golf development types, namely short courses, public-municipal golf-courses and residential golf estates, are recorded. This leaves a gap in the understanding of the Garden Route as a golfing destination, as well as opening the floor for public speculation about the status of the Garden Route golfscape. This situation emphasises the need for a description of the Garden Route golfscape in order to achieve a better understanding of it and of the Garden Route as an emerging golfing destination. The research aspires to describe the Garden Route golfscape in terms of the geographic spatial distribution and characteristics of all the golf development types mentioned. -

State of Rivers Report, the Product of a Variety of Organizations, Researchers and Scientists, Attempts to Inform Decision Makers, Interested Parties and the OREWORD
STATESTATE OFOF RIVERSRIVERS REPORTREPORT RIVERS OF THE GOURITZ WATER MANAGEMENT AREA 2007 RRIVEIVERR HHEALTEALTHH PPRROGOGRRAMMEAMME ii RIVERS OF THE GOURITZ WATER MANAGEMENT AREA 2007: SUMMARY The Gouritz Water Management Area (WMA) comprises the Goukou and Duiwenhoks, Gouritz and Garden Route rivers. Beaufort West The Gouritz River is the main river within the WMA. It originates in D the Great Karoo and enters the Indian Ocean at Gouritzmond. w y k 4HQVY[YPI\[HYPLZVM[OL.V\YP[a9P]LYHYL[OL.YVV[.HTRH a Leeu Gamka and Olifants rivers. The Goukou and Duiwenhoks rivers N1 are small rivers draining the Langeberg a Laingsburg k 4V\U[HPUZHUKÅV^V]LY[OLJVHZ[HSWSHPUZ B m u a f fe G west of Mossel Bay. The main rivers of the ls Garden Route, east of the Gouritz River, Touwsrivier Tou Olifants are the Hartenbos, Klein Brak, Groot Brak, ws Oudtshoorn Knysna, Bietou, Keurbooms, Groot and Calitzdorp G Uniondale root Bloukrans. Kammanassie George D u N2 Knysna i Land-use in the area consists largely of sheep and ostrich w G e Albertinia n G o farming in the arid Great Karoo, extensive irrigation of h o u o u r i Mossel Bay k t k z s Plettenberg o lucerne, grapes and deciduous fruit in the Little Karoo, and u Bay forestry, tourism and petrochemical industries in the coastal Stilbaai Gouritzmond belt. Indigenous forests, wetlands, lakes and estuaries of high conservation status are found in the wetter south eastern portion of the WMA. OVERALL STATE Generally, only the upper reaches of the coastal rivers and their tributaries in the WMA are still in a natural or good ecological state, while many of the lower reaches are in a good to fair state. -

Of the Garden Route
Please support our advertisers who make this free guide possible. FREE 1st Edition ‘Enamelled meads and the most beautiful pastures still added to this scene of magnificence GATEWAYGUIDES with which I was truly enchanted.’ Francois le Vaillant • intrepid explorer • on visiting Outeniqualand • 1781 PORT ELIZABETH 37 km 53 km 28 km 32 km 16 km 23 km 25 km 33 km 33 km 12 km 26 km GEORGE CAPE TOWN 50 Places Riversdale Albertinia Mossel Bay Great Brak River Bay George Wilderness Sedgefield Knysna Plettenberg Bay Nature’s Valley Bloukrans Storms River birdge Road to Oudtshoorn Road to Oudtshoorn Train Line of the Garden Route Roads to Ladismith Montagu Pass Duiwelskop Pass N Pass-to-Pass Trail Karatara 12 est 1772 (Khoi for ‘horse hill’ est 1941 for Homtini Pass ex-woodcutters) was ment to be Barrington Pass Geelhoutboomberg Barrington named after the owner of Karatara Portland River Beervlei Manor Outeniqua Bergplaas Nature reserve (start of the Sleeping Beauty Langeberg Jonkerberg Outeniqua Trail) . Hiking Trail Outeniqua Pass Outeniqua Mountains Melville Peak Robinson CradockPeak Cloetes Pass Pass 1578 m Garcia Pass 16 Rheenendal Knysna River Phantom George Peak Pass 2 Hops Farming 18 introduced in 1337 m 1935 due to Portland WWI Manor 19 Jakkalsvlei Groenkop Woodville Garcia Diep Herbertsdale River Knysna Tourism Blanco Garden Woodville Fancourt Route Big Tree Dam (800 years old) You just can’t get any closer to the sea! Ginnesville Attaquas Duiwe Luxury accommodation at Herold’s Bay on the Garden Route Road to 21 River Cape Town Kloof Pass Saasveld i (first European Geelhout- Forestry boom Trail College use 1689) i 20 17 Hoekraal River 328 404 Ruigtevlei Tel/Fax: +27 (0)44 872 9019 | Manager: +27 (0)84 544 1231 • www. -

Supplementary Valuation Roll 4
KNYSNA LOCAL MUNICIPALITY SV04 Supplementary Valuation roll for the period 1 July 2017 - 30 June 2022 Registered or other description of the property Category determined in terms of Extent of the Market value of Full Names of Owner(s) Physical address of the property Remarks Section 8 of the Act property (HA) the property Town Allotment Suburb Erf Nr Portion Unit Section SECT 78(1)(f) - THAT MUST BE REVALUED FOR ANY BELVEDERE BELVEDERE ESTATE 00000224 00000 00000 0000 BELVIDERE PARK HOME OWNERS ASSOC PUBLIC OPEN SPACES BELVIDERE ESTATE 224 - 0 0,7598 5 600 000 OTHER EXCEPTIONAL REASON SECT 78(1)(f) - THAT MUST BE REVALUED FOR ANY BELVEDERE BELVEDERE ESTATE 00000395 00000 00000 0000 KIDSON IVAN STEWART RESIDENTIAL UPPER DUTHIE DRIVE 395 - 0 0,1190 2 800 000 OTHER EXCEPTIONAL REASON BUSINESS & COMMERCIAL SECT 78(1)(c) - SUBDIVIDED OR CONSOLIDATED AFTER BELVEDERE BELVEDERE ESTATE 00000439 00000 00000 0000 TURNER MICHAEL DONALD LE SUEUR CLOSE 439 - 0 0,1859 3 200 000 PROPERTIES THE LAST GENERAL VALUATION BELVEDERE BELVEDERE ESTATE 00000471 00000 00000 0000 BARNETT DALE ANTHONY / DIEKMANN VIKTORIIA RESIDENTIAL UPPER DUTHIE DRIVE 471 - 0 0,1395 4 900 000 SECT 78(1)(g) - OF WHICH THE CATEGORY HAS CHANGED SECT 78(1)(d) - OF WHICH THE MARKET VALUE HAS BELVEDERE BELVEDERE ESTATE 00000516 00000 00000 0000 POWAPROPS 50 PTY LTD RESIDENTIAL CURRY CLOSE 516 - 0 0,1222 3 000 000 SUBSTANTIALLY INCREASED OR DECREASED FOR ANY REASON AFTER THE LAST GENERAL VALUATION BUSINESS & COMMERCIAL BELVEDERE BELVEDERE ESTATE 00000527 00000 00000 0000 COLUMBIA PARK -

Section B: Activity Information
Final Draft Basic Assessment Report for the Proposed Development of x2 Dwelling Units & Associated Infrastructure On Portion 49 & 50 of the Farm Uitzicht 216, Knysna Reference No: NEAS REF: DEA/EIA/0001554/2012 DEA REF NR. 14/12/16/3/3/1/755 BOKAMOSO December 2013 LANDSCAPE ARCHITECTS & ENVIRONMENTALCONSULTANTS P.O. BOX 11375 MAROELANA 0161 TEL: (012) 346 3810 Fax: 086 570 5659 Email:[email protected] UITZICHT 216 KNYSNA BASIC ASSESSMENT REPORT (For official use only) File Reference Number: Application Number: Date Received: Basic assessment report in terms of the Environmental Impact Assessment Regulations, 2010, promulgated in terms of the National Environmental Management Act, 1998 (Act No. 107 of 1998), as amended. Kindly note that: 1. This basic assessment report is a standard report that may be required by a competent authority in terms of the EIA Regulations, 2010 and is meant to streamline applications. Please make sure that it is the report used by the particular competent authority for the activity that is being applied for. 2. This report format is current as of 1 September 2012. It is the responsibility of the applicant to ascertain whether subsequent versions of the form have been published or produced by the competent authority 3. The report must be typed within the spaces provided in the form. The size of the spaces provided is not necessarily indicative of the amount of information to be provided. The report is in the form of a table that can extend itself as each space is filled with typing. 4. Where applicable tick the boxes that are applicable in the report.