Checklist of Myxomycetes from India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
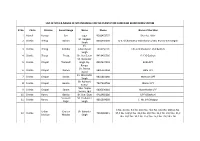
List of Sites & Names of Site Incharge for The
LIST OF SITES & NAMES OF SITE INCHARGE FOR THE PLANTATION CAMPAIGN MONITORING SYSTEM Sl No Circle Division Forest Range Name Phone Name of the Sites 1 Mandi Karsog Seri sagar 9560453757 Shamlat Haler Sh. Ranjeet 2 Shimla Theog Balson 8091350003 U-371 Chamble,U-366 Khanar,U-401 Khar,U-370 Kargoli Singh Sh. 3 Shimla Theog Kotkhai Ghanshyam 7018556195 UPF-479 Khola,UPF 456 Badruni Singh 4 Shimla Theog Theog Sh. Hari Saran 9418455366 D-126 Guthan Sh. Narender 5 Shimla Chopal Tharoach Singh, Dy. 8894537991 Birda DPF Ranger Sh. Parma 6 Shimla Chopal Nerwa 9805161004 Obta UPF Nand Sh. Mahender 7 Shimla Chopal Kanda 9816601060 Malnoon DPF Singh Sh. Ashwani 8 Shimla Chopal Bamta 7807503756 Momvi UPF Kumar Miss. Sapna 9 Shimla Chopal Sarain 9805198318 Nora-thalan DPF Verma, Fgd 10 Shimla Rohru Bashla Sh. Sunil Dutt 9418469089 UPF-8 Bashuni Sarswati Sh. Yashwant 11 Shimla Rohru 9816364369 C. No. 14 Chhajpur Nagar Singh C No. 2,C No. 5,C No. 10,C No. 40,C No. 12,C No. 16(b),C No. Urban Chaura Sh. Bahadur 12 Shimla 7018582415 37,C No. 16(c),C No. 36,C No. 19,C No. 34,C No. 21,C No. 31,C Division Maidan Singh No. 24,C No. 30,C No. 25,C No. 26,C No. 29,C No. 28 13 Hamirpur Una Bharwain Piar Singh FR 9418177107 R-II Lohara AC-4 Ashok Kumar 14 Hamirpur Una Amb 9418137656 R-III-Dharuhi-DC-1,Shamlat Haler FR Rajesh Kumar 15 Hamirpur Una Una 9418156944 SL Kotla Kalan,SL Badhera,SL Barnoh FR Ajeet Singh 16 Hamirpur Una Bangana 9816112244 U.P.F. -

Delineation of Suitable Areas for Potential Temperate Fruit Crops In
Journal of Pharmacognosy and Phytochemistry 2019; 8(3): 4695-4703 E-ISSN: 2278-4136 P-ISSN: 2349-8234 JPP 2019; 8(3): 4695-4703 Delineation of suitable areas for potential Received: 22-03-2019 Accepted: 23-04-2019 temperate fruit crops in Himachal Pradesh under changing climatic conditions Aditya Chandigarh University, Gharuan, Mohali, Punjab, India Aditya and SK Bhardwaj SK Bhardwaj Dr. Y.S. Parmar, University of Abstract Horticulture and Forestry, The climatic changes with respect to rise in temperature, erratic precipitation and lack of chilling hours Nauni, Solan, India have affected the hill horticultural production system adversely. A study was conducted to identify the climatic suitability for optimum production of major temperate fruit crops in high and mid hills of Himachal Pradesh. Using Geographical Information System (GIS), the suitability analyses were performed with digital processing of geo-referenced data on topography, climate, soil and land cover. The potential production areas for each crop were identified by ArcGIS spatial analyst. The study revealed that the higher reaches such as Manali, Bhang, Naggar and Raisan in Kullu; Kotgarh, Jubbal, Kumarsain, Chopal and Rohru of Shimla and Kinnaur district covering Sangla, Kalpa, Sharbo and Pooh were highly suitable for growing apple. Whereas, the areas comprising of Bajaura and Bhuntar in Kullu; Karsog, Nagwain and Jhanjheli in Mandi; Theog area in lower parts of Shimla district were moderately suitable for apple cultivation. The suitability map for sub temperate fruit crops indicated that Kandaghat, Kunihar and Dharampur in Solan and Rajgarh area of Sirmaur district were highly suitable for peach, plum and apricot. However, Nalagarh in Solan and Rohru in Shimla district have become marginally suitable zones for sub temperate fruit crops. -

Myxomycetes NMW 2012Orange, Updated KS 2017.Docx
Myxomycete (Slime Mould) Collection Amgueddfa Cymru-National Museum Wales (NMW) Alan Orange (2012), updated by Katherine Slade (2017) Myxomycetes (true or plasmodial slime moulds) belong to the Eumycetozoa, within the Amoebozoa, a group of eukaryotes that are basal to a clade containing animals and fungi. Thus although they have traditionally been studied by mycologists they are distant from the true fungi. Arrangement & Nomenclature Slime Mould specimens in NMW are arranged in alphabetical order of the currently accepted name (as of 2012). Names used on specimen packets that are now synonyms are cross referenced in the list below. The collection currently contains 157 Myxomycete species. Specimens are mostly from Britain, with a few from other parts of Europe or from North America. The current standard work for identification of the British species is: Ing, B. 1999. The Myxomycetes of Britain and Ireland. An Identification Handbook. Slough: Richmond Publishing Co. Ltd. Nomenclature follows the online database of Slime Mould names at www.eumycetozoa.com (accessed 2012). This database is largely in line with Ing (1999). Preservation The feeding stage is a multinucleate motile mass known as a plasmodium. The fruiting stage is a dry, fungus-like structure containing abundant spores. Mature fruiting bodies of Myxomycetes can be collected and dried, and with few exceptions (such as Ceratiomyxa) they preserve well. Plasmodia cannot be preserved, but it is useful to record the colour if possible. Semi-mature fruiting bodies may continue to mature if collected with the substrate and kept in a cool moist chamber. Collected plasmodia are unlikely to fruit. Specimens are stored in boxes to prevent crushing; labels should not be allowed to touch the specimen. -

Economic Survey of Himachal Pradesh 2013-14
ECONOMIC SURVEY OF HIMACHAL PRADESH 2013-14 Economics & Statistics Department FOREWORD Economic Survey is one of the budget documents which indicates the important economic activities and achievements of the Government. The salient features of the State of the economy of Himachal Pradesh during 2013-14 are presented in Part-I, and statistical tables on various subjects are given in Part-II. I am thankful to all the departments and public undertakings for their co-operation in making available the material included in the Survey. The burden of collection and updating the huge and voluminous data and its presentation in a concise and inter-related form was borne by the Economics & Statistics Department. I appreciate and commend the work done by the officers and officials of this department. Dr. Shrikant Baldi Principal Secretary (Finance, Plg., and Eco. & Stat.) to the Govt.of Himachal Pradesh. I N D E X Contents Pages 1. General Review 1 2. State Income and Public Finance 10 3. Institutional and Bank Finances 14 4. Excise and Taxation 29 5. Price Movement 32 6. Food Security and Civil Supplies 34 7. Agriculture and Horticulture 39 8. Animal Husbandry and Fisheries 52 9. Forest and Environment 61 10. Water Resource Management 65 11. Industries and Mining 67 12. Labour and Employment 70 13. Power 74 14. Transport and Communication 101 15. Tourism and Civil Aviation 106 16. Education 110 17. Health 124 18. Social Welfare Programme 130 19. Rural Development 141 20. Housing and Urban Development 147 21. Panchayati Raj 152 22. Information and Science Technology 155 Part-I ECONOMIC SURVEY-2013-14 1 GENERAL REVIEW Economic Situation at National Level 1.1 THE Indian economy has estimated at ' 93.90 lakh crore as experienced a slowdown for the past against ' 83.90 lakh crore in 2011-12 two years and country is passing showing an increase of 11.9 percent through a difficult phase caused by the during the year. -

The Fungi of Slapton Ley National Nature Reserve and Environs
THE FUNGI OF SLAPTON LEY NATIONAL NATURE RESERVE AND ENVIRONS APRIL 2019 Image © Visit South Devon ASCOMYCOTA Order Family Name Abrothallales Abrothallaceae Abrothallus microspermus CY (IMI 164972 p.p., 296950), DM (IMI 279667, 279668, 362458), N4 (IMI 251260), Wood (IMI 400386), on thalli of Parmelia caperata and P. perlata. Mainly as the anamorph <it Abrothallus parmeliarum C, CY (IMI 164972), DM (IMI 159809, 159865), F1 (IMI 159892), 2, G2, H, I1 (IMI 188770), J2, N4 (IMI 166730), SV, on thalli of Parmelia carporrhizans, P Abrothallus parmotrematis DM, on Parmelia perlata, 1990, D.L. Hawksworth (IMI 400397, as Vouauxiomyces sp.) Abrothallus suecicus DM (IMI 194098); on apothecia of Ramalina fustigiata with st. conid. Phoma ranalinae Nordin; rare. (L2) Abrothallus usneae (as A. parmeliarum p.p.; L2) Acarosporales Acarosporaceae Acarospora fuscata H, on siliceous slabs (L1); CH, 1996, T. Chester. Polysporina simplex CH, 1996, T. Chester. Sarcogyne regularis CH, 1996, T. Chester; N4, on concrete posts; very rare (L1). Trimmatothelopsis B (IMI 152818), on granite memorial (L1) [EXTINCT] smaragdula Acrospermales Acrospermaceae Acrospermum compressum DM (IMI 194111), I1, S (IMI 18286a), on dead Urtica stems (L2); CY, on Urtica dioica stem, 1995, JLT. Acrospermum graminum I1, on Phragmites debris, 1990, M. Marsden (K). Amphisphaeriales Amphisphaeriaceae Beltraniella pirozynskii D1 (IMI 362071a), on Quercus ilex. Ceratosporium fuscescens I1 (IMI 188771c); J1 (IMI 362085), on dead Ulex stems. (L2) Ceriophora palustris F2 (IMI 186857); on dead Carex puniculata leaves. (L2) Lepteutypa cupressi SV (IMI 184280); on dying Thuja leaves. (L2) Monographella cucumerina (IMI 362759), on Myriophyllum spicatum; DM (IMI 192452); isol. ex vole dung. (L2); (IMI 360147, 360148, 361543, 361544, 361546). -

Myxomycetes of Taiwan XXV. the Family Stemonitaceae
Taiwania, 59(3): 210‒219, 2014 DOI: 10.6165/tai.2014.59.210 RESEARCH ARTICLE Myxomycetes of Taiwan XXV. The Family Stemonitaceae Chin-Hui Liu* and Jong-How Chang Institute of Plant Science, National Taiwan University, Taipei, Taiwan 106, R.O.C. * Corresponding author. Email: [email protected] (Manuscript received 22 February 2014; accepted 30 May 2014) ABSTRACT: Species of ten genera of Stemonitaceae, including Collaria, Comatricha, Enerthenema, Lamproderma, Macbrideola, Paradiacheopsis, Stemonaria, Stemonitis, Stemonitopsis, and Symphytocarpus, collected from Taiwan are critically revised. Of the 42 species recorded, Enerthenema intermedium and Stemonitopsis subcaespitosa are new to Taiwan, thus are described and illustrated in this paper. Keys to the species of all genera, and to the genera of the family are also provided. KEY WORDS: Myxomycetes, Stemonitaceae, Taiwan, taxonomy. INTRODUCTION 4’. Fruiting body more than 0.5 mm tall; sporangia cylindrical …..... 5 5. Outermost branches of capillitium united to form a delicate, complete surface net ………………………...…………. Stemonitis The family Stemonitaceae is a monotypic family of 5’. No surface net ………………………………………... Stemonaria the order Stemonitales. It contains 16 genera and 202 6. Peridium persistent, usually iridescent …………….. Lamproderma species in the world (Lado, 2005–2013). In this paper 6’. Peridium disappearing in mature fruiting bodies, at most leaving a collar or a few flakes ……………………………………………... 7 we present a list of 40 taxa including their ecological 7. Capillitium sparse, not anastomosing, with few branches ………… data compiled from the previous records of this family …………………………………………..……….. Paradiacheopsis in Taiwan and 2 new records of Taiwan, Enerthenema 7’. Capillitium usually abundant, anastomosing ……………….....… 8 intermedium and Stemonitopsis subcaespitosa. 8. Surface net of capillitium present, over at least the lower portion; sporangia cylindrical ……………………………….. -

Yüzüncü Yıl Üniversitesi Fen Bilimleri Enstitüsü Dergisi
Yüzüncü Yıl ÜniversitesiFen Bilimleri Enstitüsü Dergisi Cilt 26, Sayı 1 (Nisan), 1-10, 2021 Yüzüncü Yıl Üniversitesi Fen Bilimleri Enstitüsü Dergisi http://dergipark.gov.tr/yyufbed Research Article (Araştırma Makalesi) Myxomycetes Growing on Culture Logs Pleurotus ostreatus (Jacq.) P. Kumm. and Lentinula edodes (Berk.) Pegler Gönül EROĞLU*1, Sinan ALKAN2, Gıyasettin KAŞIK1 1Selçuk University, Faculty of Science, Department of Biology, 42130, Konya, Turkey 2Selçuk University, Çumra School of Applied Sciences, Organic Agriculture Administration Department, 42500, Konya, Turkey Gönül EROĞLU, ORCID No: 0000-0001-6323-2077, Sinan ALKAN, ORCID No: 0000-0001-7725-1957, Gıyasettin KAŞIK, ORCID No: 0000-0001-8304-6554 *Corresponding author e-mail: [email protected] Article Info Abstract: In this study, it was aimed to identify myxomycetes that develop on natural and synthetic logs used in culture mushroom cultivation. For this study, the logs brought Received: 17.07.2020 from three different regions (Sızma village-Konya, Hadim-Konya, Yenice-Karabük) in Accepted: 22.02.2021 2015 and the synthetic logs were applied the procedure required for culture mushroom Published April 2021 cultivation and then the spawn of Pleurotus ostreatus (Jacq.) P. Kumm. and Lentinula DOI: edodes (Berk.) Pegler were inoculated to the logs. The inoculated logs were taken to the Keywords mushroom growing room where climatic conditions such as humidity, temperature and Cultivated mushroom, lighting were provided automatically. While checking the growth of the cultivated Myxomycetes, fungi, it was observed that the myxomycetes plasmodium and sporocarp also developed Moist chamber culture on the culture logs. Myxomycetes develop on organic plant debris, which is their natural environment, and are also developed in the laboratory using the moist chamber technique. -

Slime Moulds
Queen’s University Biological Station Species List: Slime Molds The current list has been compiled by Richard Aaron, a naturalist and educator from Toronto, who has been running the Fabulous Fall Fungi workshop at QUBS between 2009 and 2019. Dr. Ivy Schoepf, QUBS Research Coordinator, edited the list in 2020 to include full taxonomy and information regarding species’ status using resources from The Natural Heritage Information Centre (April 2018) and The IUCN Red List of Threatened Species (February 2018); iNaturalist and GBIF. Contact Ivy to report any errors, omissions and/or new sightings. Based on the aforementioned criteria we can expect to find a total of 33 species of slime molds (kingdom: Protozoa, phylum: Mycetozoa) present at QUBS. Species are Figure 1. One of the most commonly encountered reported using their full taxonomy; common slime mold at QUBS is the Dog Vomit Slime Mold (Fuligo septica). Slime molds are unique in the way name and status, based on whether the species is that they do not have cell walls. Unlike fungi, they of global or provincial concern (see Table 1 for also phagocytose their food before they digest it. details). All species are considered QUBS Photo courtesy of Mark Conboy. residents unless otherwise stated. Table 1. Status classification reported for the amphibians of QUBS. Global status based on IUCN Red List of Threatened Species rankings. Provincial status based on Ontario Natural Heritage Information Centre SRank. Global Status Provincial Status Extinct (EX) Presumed Extirpated (SX) Extinct in the -

Biodiversity of Plasmodial Slime Moulds (Myxogastria): Measurement and Interpretation
Protistology 1 (4), 161–178 (2000) Protistology August, 2000 Biodiversity of plasmodial slime moulds (Myxogastria): measurement and interpretation Yuri K. Novozhilova, Martin Schnittlerb, InnaV. Zemlianskaiac and Konstantin A. Fefelovd a V.L.Komarov Botanical Institute of the Russian Academy of Sciences, St. Petersburg, Russia, b Fairmont State College, Fairmont, West Virginia, U.S.A., c Volgograd Medical Academy, Department of Pharmacology and Botany, Volgograd, Russia, d Ural State University, Department of Botany, Yekaterinburg, Russia Summary For myxomycetes the understanding of their diversity and of their ecological function remains underdeveloped. Various problems in recording myxomycetes and analysis of their diversity are discussed by the examples taken from tundra, boreal, and arid areas of Russia and Kazakhstan. Recent advances in inventory of some regions of these areas are summarised. A rapid technique of moist chamber cultures can be used to obtain quantitative estimates of myxomycete species diversity and species abundance. Substrate sampling and species isolation by the moist chamber technique are indispensable for myxomycete inventory, measurement of species richness, and species abundance. General principles for the analysis of myxomycete diversity are discussed. Key words: slime moulds, Mycetozoa, Myxomycetes, biodiversity, ecology, distribu- tion, habitats Introduction decay (Madelin, 1984). The life cycle of myxomycetes includes two trophic stages: uninucleate myxoflagellates General patterns of community structure of terrestrial or amoebae, and a multi-nucleate plasmodium (Fig. 1). macro-organisms (plants, animals, and macrofungi) are The entire plasmodium turns almost all into fruit bodies, well known. Some mathematics methods are used for their called sporocarps (sporangia, aethalia, pseudoaethalia, or studying, from which the most popular are the quantita- plasmodiocarps). -

What Substrate Cultures Can Reveal: Myxomycetes and Myxomycete-Like Organisms from the Sultanate of Oman
Mycosphere 6 (3): 356–384(2015) ISSN 2077 7019 www.mycosphere.org Article Mycosphere Copyright © 2015 Online Edition Doi 10.5943/mycosphere/6/3/11 What substrate cultures can reveal: Myxomycetes and myxomycete-like organisms from the Sultanate of Oman Schnittler M1, Novozhilov YK2, Shadwick JDL3, Spiegel FW3, García-Carvajal E4, König P1 1Institute of Botany and Landscape Ecology, Ernst Moritz Arndt University Greifswald, Soldmannstr. 15, D-17487 Greifswald, Germany 2V.L. Komarov Botanical Institute of the Russian Academy of Sciences, Prof. Popov St. 2, 197376 St. Petersburg, Russia 3University of Arkansas, Department of Biological Sciences, SCEN 601, 1 University of Arkansas, Fayetteville, AR 72701, USA 4Royal Botanic Garden (CSIC), Plaza de Murillo, 2, Madrid, E-28014, Spain Schnittler M, Novozhilov YK, Shadwick JDL, Spiegel FW, García-Carvajal E, König P 2015 – What substrate cultures can reveal: Myxomycetes and myxomycete-like organisms from the Sultanate of Oman. Mycosphere 6(3), 356–384, doi 10.5943/mycosphere/6/3/11 Abstract A total of 299 substrate samples collected throughout the Sultanate of Oman were analyzed for myxomycetes and myxomycete-like organisms (MMLO) with a combined approach, preparing one moist chamber culture and one agar culture for each sample. We recovered 8 forms of Myxobacteria, 2 sorocarpic amoebae (Acrasids), 19 known and 6 unknown taxa of protostelioid amoebae (Protostelids), and 50 species of Myxomycetes. Moist chambers and agar cultures completed each other. No method alone can detect the whole diversity of myxomycetes as the most species-rich group of MMLO. A significant overlap between the two methods was observed only for Myxobacteria and some myxomycetes with small sporocarps. -

9B Taxonomy to Genus
Fungus and Lichen Genera in the NEMF Database Taxonomic hierarchy: phyllum > class (-etes) > order (-ales) > family (-ceae) > genus. Total number of genera in the database: 526 Anamorphic fungi (see p. 4), which are disseminated by propagules not formed from cells where meiosis has occurred, are presently not grouped by class, order, etc. Most propagules can be referred to as "conidia," but some are derived from unspecialized vegetative mycelium. A significant number are correlated with fungal states that produce spores derived from cells where meiosis has, or is assumed to have, occurred. These are, where known, members of the ascomycetes or basidiomycetes. However, in many cases, they are still undescribed, unrecognized or poorly known. (Explanation paraphrased from "Dictionary of the Fungi, 9th Edition.") Principal authority for this taxonomy is the Dictionary of the Fungi and its online database, www.indexfungorum.org. For lichens, see Lecanoromycetes on p. 3. Basidiomycota Aegerita Poria Macrolepiota Grandinia Poronidulus Melanophyllum Agaricomycetes Hyphoderma Postia Amanitaceae Cantharellales Meripilaceae Pycnoporellus Amanita Cantharellaceae Abortiporus Skeletocutis Bolbitiaceae Cantharellus Antrodia Trichaptum Agrocybe Craterellus Grifola Tyromyces Bolbitius Clavulinaceae Meripilus Sistotremataceae Conocybe Clavulina Physisporinus Trechispora Hebeloma Hydnaceae Meruliaceae Sparassidaceae Panaeolina Hydnum Climacodon Sparassis Clavariaceae Polyporales Gloeoporus Steccherinaceae Clavaria Albatrellaceae Hyphodermopsis Antrodiella -

Slime Molds: Biology and Diversity
Glime, J. M. 2019. Slime Molds: Biology and Diversity. Chapt. 3-1. In: Glime, J. M. Bryophyte Ecology. Volume 2. Bryological 3-1-1 Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 18 July 2020 and available at <https://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 3-1 SLIME MOLDS: BIOLOGY AND DIVERSITY TABLE OF CONTENTS What are Slime Molds? ....................................................................................................................................... 3-1-2 Identification Difficulties ...................................................................................................................................... 3-1- Reproduction and Colonization ........................................................................................................................... 3-1-5 General Life Cycle ....................................................................................................................................... 3-1-6 Seasonal Changes ......................................................................................................................................... 3-1-7 Environmental Stimuli ............................................................................................................................... 3-1-13 Light .................................................................................................................................................... 3-1-13 pH and Volatile Substances