Genome-Wide Diversity and Gene Expression Profiling of Babesia Microti Isolates Identify Polymorphic Genes That Mediate Host-Pathogen Interactions J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analysis of Gene Expression Data for Gene Ontology
ANALYSIS OF GENE EXPRESSION DATA FOR GENE ONTOLOGY BASED PROTEIN FUNCTION PREDICTION A Thesis Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Master of Science Robert Daniel Macholan May 2011 ANALYSIS OF GENE EXPRESSION DATA FOR GENE ONTOLOGY BASED PROTEIN FUNCTION PREDICTION Robert Daniel Macholan Thesis Approved: Accepted: _______________________________ _______________________________ Advisor Department Chair Dr. Zhong-Hui Duan Dr. Chien-Chung Chan _______________________________ _______________________________ Committee Member Dean of the College Dr. Chien-Chung Chan Dr. Chand K. Midha _______________________________ _______________________________ Committee Member Dean of the Graduate School Dr. Yingcai Xiao Dr. George R. Newkome _______________________________ Date ii ABSTRACT A tremendous increase in genomic data has encouraged biologists to turn to bioinformatics in order to assist in its interpretation and processing. One of the present challenges that need to be overcome in order to understand this data more completely is the development of a reliable method to accurately predict the function of a protein from its genomic information. This study focuses on developing an effective algorithm for protein function prediction. The algorithm is based on proteins that have similar expression patterns. The similarity of the expression data is determined using a novel measure, the slope matrix. The slope matrix introduces a normalized method for the comparison of expression levels throughout a proteome. The algorithm is tested using real microarray gene expression data. Their functions are characterized using gene ontology annotations. The results of the case study indicate the protein function prediction algorithm developed is comparable to the prediction algorithms that are based on the annotations of homologous proteins. -

Molecular and Physiological Basis for Hair Loss in Near Naked Hairless and Oak Ridge Rhino-Like Mouse Models: Tracking the Role of the Hairless Gene
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2006 Molecular and Physiological Basis for Hair Loss in Near Naked Hairless and Oak Ridge Rhino-like Mouse Models: Tracking the Role of the Hairless Gene Yutao Liu University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Life Sciences Commons Recommended Citation Liu, Yutao, "Molecular and Physiological Basis for Hair Loss in Near Naked Hairless and Oak Ridge Rhino- like Mouse Models: Tracking the Role of the Hairless Gene. " PhD diss., University of Tennessee, 2006. https://trace.tennessee.edu/utk_graddiss/1824 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Yutao Liu entitled "Molecular and Physiological Basis for Hair Loss in Near Naked Hairless and Oak Ridge Rhino-like Mouse Models: Tracking the Role of the Hairless Gene." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Life Sciences. Brynn H. Voy, Major Professor We have read this dissertation and recommend its acceptance: Naima Moustaid-Moussa, Yisong Wang, Rogert Hettich Accepted for the Council: Carolyn R. -

The N-Cadherin Interactome in Primary Cardiomyocytes As Defined Using Quantitative Proximity Proteomics Yang Li1,*, Chelsea D
© 2019. Published by The Company of Biologists Ltd | Journal of Cell Science (2019) 132, jcs221606. doi:10.1242/jcs.221606 TOOLS AND RESOURCES The N-cadherin interactome in primary cardiomyocytes as defined using quantitative proximity proteomics Yang Li1,*, Chelsea D. Merkel1,*, Xuemei Zeng2, Jonathon A. Heier1, Pamela S. Cantrell2, Mai Sun2, Donna B. Stolz1, Simon C. Watkins1, Nathan A. Yates1,2,3 and Adam V. Kwiatkowski1,‡ ABSTRACT requires multiple adhesion, cytoskeletal and signaling proteins, The junctional complexes that couple cardiomyocytes must transmit and mutations in these proteins can cause cardiomyopathies (Ehler, the mechanical forces of contraction while maintaining adhesive 2018). However, the molecular composition of ICD junctional homeostasis. The adherens junction (AJ) connects the actomyosin complexes remains poorly defined. – networks of neighboring cardiomyocytes and is required for proper The core of the AJ is the cadherin catenin complex (Halbleib and heart function. Yet little is known about the molecular composition of the Nelson, 2006; Ratheesh and Yap, 2012). Classical cadherins are cardiomyocyte AJ or how it is organized to function under mechanical single-pass transmembrane proteins with an extracellular domain that load. Here, we define the architecture, dynamics and proteome of mediates calcium-dependent homotypic interactions. The adhesive the cardiomyocyte AJ. Mouse neonatal cardiomyocytes assemble properties of classical cadherins are driven by the recruitment of stable AJs along intercellular contacts with organizational and cytosolic catenin proteins to the cadherin tail, with p120-catenin β structural hallmarks similar to mature contacts. We combine (CTNND1) binding to the juxta-membrane domain and -catenin β quantitative mass spectrometry with proximity labeling to identify the (CTNNB1) binding to the distal part of the tail. -
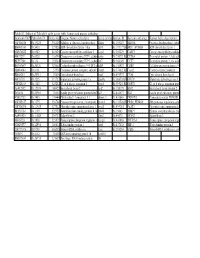
Table S3: Subset of Zebrafish Early Genes with Human And
Table S3: Subset of Zebrafish early genes with human and mouse orthologs Genbank ID(ZFZebrafish ID Entrez GenUnigene Name (zebrafish) Gene symbo Human ID Humann ortholog Human Gene description AW116838 Dr.19225 336425 Aldolase a, fructose-bisphosphate aldoa Hs.155247 ALDOA Fructose-bisphosphate aldola BM005100 Dr.5438 327026 ADP-ribosylation factor 1 like arf1l Hs.119177||HsARF1_HUMAN ADP-ribosylation factor 1 AW076882 Dr.6582 403025 Cancer susceptibility candidate 3 casc3 Hs.350229 CASC3 Cancer susceptibility candidat AI437239 Dr.6928 116994 Chaperonin containing TCP1, subun cct6a Hs.73072||Hs.CCT6A T-complex protein 1, zeta sub BE557308 Dr.134 192324 Chaperonin containing TCP1, subun cct7 Hs.368149 CCT7 T-complex protein 1, eta subu BG303647 Dr.26326 321602 Cyclin-dependent kinase 9 (CDC2-recdk9 Hs.150423 CDK9 Cell division protein kinase 9 AB040044 Dr.8161 57970 Coatomer protein complex, subunit zcopz1 Hs.37482||Hs.Copz2 Coatomer zeta-2 subunit BI888253 Dr.20911 30436 Eyes absent homolog 1 eya1 Hs.491997 EYA4 Eyes absent homolog 4 AI878758 Dr.3225 317737 Glutamate dehydrogenase 1a glud1a Hs.368538||HsGLUD1 Glutamate dehydrogenase 1, AW128619 Dr.1388 325284 G1 to S phase transition 1 gspt1 Hs.59523||Hs.GSPT1 G1 to S phase transition prote AF412832 Dr.12595 140427 Heat shock factor 2 hsf2 Hs.158195 HSF2 Heat shock factor protein 2 D38454 Dr.20916 30151 Insulin gene enhancer protein Islet3 isl3 Hs.444677 ISL2 Insulin gene enhancer protein AY052752 Dr.7485 170444 Pbx/knotted 1 homeobox 1.1 pknox1.1 Hs.431043 PKNOX1 Homeobox protein PKNOX1 -

A Rare Variant in MCF2L Identified Using Exclusion Linkage in A
European Journal of Human Genetics (2016) 24, 86–91 & 2016 Macmillan Publishers Limited All rights reserved 1018-4813/16 www.nature.com/ejhg ARTICLE A rare variant in MCF2L identified using exclusion linkage in a pedigree with premature atherosclerosis Stephanie Maiwald1,7, Mahdi M Motazacker1,7,8, Julian C van Capelleveen1, Suthesh Sivapalaratnam1, Allard C van der Wal2, Chris van der Loos2, John JP Kastelein1, Willem H Ouwehand3,4, G Kees Hovingh1, Mieke D Trip1,5, Jaap D van Buul6 and Geesje M Dallinga-Thie*,1 Cardiovascular disease (CVD) is a major cause of death in Western societies. CVD risk is largely genetically determined. The molecular pathology is, however, not elucidated in a large number of families suffering from CVD. We applied exclusion linkage analysis and next-generation sequencing to elucidate the molecular defect underlying premature CVD in a small pedigree, comprising two generations of which six members suffered from premature CVD. A total of three variants showed co-segregation with the disease status in the family. Two of these variants were excluded from further analysis based on the prevalence in replication cohorts, whereas a non-synonymous variant in MCF.2 Cell Line Derived Transforming Sequence-like protein (MCF2L, c.2066A4G; p.(Asp689Gly); NM_001112732.1), located in the DH domain, was only present in the studied family. MCF2L is a guanine exchange factor that potentially links pathways that signal through Rac1 and RhoA. Indeed, in HeLa cells, MCF2L689Gly failed to activate Rac1 as well as RhoA, resulting in impaired stress fiber formation. Moreover, MCF2L protein was found in human atherosclerotic lesions but not in healthy tissue segments. -

1 Title: Re-Annotation of the Theileria Parva Genome Refines 53% of the Proteome And
bioRxiv preprint doi: https://doi.org/10.1101/749366; this version posted August 31, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Title: Re-annotation of the Theileria parva genome refines 53% of the proteome and 2 uncovers essential components of N-glycosylation, a conserved pathway in many 3 organisms 4 Kyle Tretina1, Roger Pelle2, Joshua Orvis1, Hanzel T. Gotia1, Olukemi O. Ifeonu1, Priti 5 Kumari1, Nicholas C. Palmateer1, Shaikh B.A. Iqbal1, Lindsay Fry3,4, Vishvanath M. 6 Nene5, Claudia Daubenberger6, Richard P. Bishop3, Joana C. Silva1,7* 7 Affiliations: 8 1Institute for Genome Sciences, University of Maryland School of Medicine, Baltimore, 9 MD, USA 10 2Biosciences eastern and central Africa-International Livestock Research Institute, 11 Nairobi, Kenya 12 3Animal Disease Research Unit, Agricultural Research Service, USDA, Pullman, WA 13 99164-7030, USA 14 4Department of Veterinary Microbiology & Pathology, Washington State University 15 Pullman, WA 99164-7040, USA 16 5International Livestock Research Institute, Nairobi, Kenya 17 6Swiss Tropical and Public Health Institute, Basel, Switzerland & University of Basel, 18 Basel, Switzerland 19 7Department of Microbiology and Immunology, University of Maryland School of 20 Medicine, Baltimore, MD, USA 21 *Corresponding author 22 23 1 bioRxiv preprint doi: https://doi.org/10.1101/749366; this version posted August 31, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 24 Abstract (<350 words) 25 Background: Genome annotation remains a significant challenge because of limitations in 26 the quality and quantity of the data being used to inform the location and function of 27 protein-coding genes and, when RNA data are used, the underlying biological complexity 28 of the processes involved in gene expression. -

Strains Used in Whole Organism Plasmodium Falciparum Vaccine Trials Differ in 2 Genome Structure, Sequence, and Immunogenic Potential 3 4 Authors: Kara A
bioRxiv preprint doi: https://doi.org/10.1101/684175; this version posted June 27, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Title: Strains used in whole organism Plasmodium falciparum vaccine trials differ in 2 genome structure, sequence, and immunogenic potential 3 4 Authors: Kara A. Moser1‡, Elliott F. Drábek1, Ankit Dwivedi1, Jonathan Crabtree1, Emily 5 M. Stucke2, Antoine Dara2, Zalak Shah2, Matthew Adams2, Tao Li3, Priscila T. 6 Rodrigues4, Sergey Koren5, Adam M. Phillippy5, Amed Ouattara2, Kirsten E. Lyke2, Lisa 7 Sadzewicz1, Luke J. Tallon1, Michele D. Spring6, Krisada Jongsakul6, Chanthap Lon6, 8 David L. Saunders6¶, Marcelo U. Ferreira4, Myaing M. Nyunt2§, Miriam K. Laufer2, Mark 9 A. Travassos2, Robert W. Sauerwein7, Shannon Takala-Harrison2, Claire M. Fraser1, B. 10 Kim Lee Sim3, Stephen L. Hoffman3, Christopher V. Plowe2§, Joana C. Silva1,8 11 12 Corresponding author: Joana C. Silva, [email protected] 13 Affiliations: 14 1 Institute for Genome Sciences, University of Maryland School of Medicine, Baltimore, 15 MD, 21201 16 2 Center for Vaccine Development and Global Health, University of Maryland School of 17 Medicine, Baltimore, MD, 21201 18 3 Sanaria, Inc. Rockville, Maryland, 20850 19 4 Department of Parasitology, Institute of Biomedical Sciences, University of São Paulo, 20 São Paulo, Brazil 21 5 Genome Informatics Section, Computational and Statistical Genomics Branch, 22 National Human -

Validation of the PARVA C.392A<T Variant in a South African Family
Validation of the PARVA c.392A>T variant in a South African family with severe Arrhythmogenic Right Ventricular Cardiomyopathy Stephen Kamuli Thesis presented for the Degree of MASTER OF SCIENCE In the Department of Medicine UniversityUNIVERSITY OF of CAPE Cape TOWN Town Supervisor: Dr Gasnat Shaboodien Co-supervisor: Professor Bongani Mayosi August 2016 i The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town DECLARATION I, …Stephen Kamuli………………, hereby declare that the work on which this dissertation/thesis is based is my original work (except where acknowledgements indicate otherwise) and that neither the whole work nor any part of it has been, is being, or is to be submitted for another degree in this or any other university. I empower the university to reproduce for the purpose of research either the whole or any portion of the contents in any manner whatsoever. Signature: signature removed Date: 18 August 2016 ii TABLE OF CONTENTS TABLE OF CONTENTS ...................................................................................................................................... I ABSTRACT .................................................................................................................................................... -

Mclean, Chelsea.Pdf
COMPUTATIONAL PREDICTION AND EXPERIMENTAL VALIDATION OF NOVEL MOUSE IMPRINTED GENES A Dissertation Presented to the Faculty of the Graduate School of Cornell University In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by Chelsea Marie McLean August 2009 © 2009 Chelsea Marie McLean COMPUTATIONAL PREDICTION AND EXPERIMENTAL VALIDATION OF NOVEL MOUSE IMPRINTED GENES Chelsea Marie McLean, Ph.D. Cornell University 2009 Epigenetic modifications, including DNA methylation and covalent modifications to histone tails, are major contributors to the regulation of gene expression. These changes are reversible, yet can be stably inherited, and may last for multiple generations without change to the underlying DNA sequence. Genomic imprinting results in expression from one of the two parental alleles and is one example of epigenetic control of gene expression. So far, 60 to 100 imprinted genes have been identified in the human and mouse genomes, respectively. Identification of additional imprinted genes has become increasingly important with the realization that imprinting defects are associated with complex disorders ranging from obesity to diabetes and behavioral disorders. Despite the importance imprinted genes play in human health, few studies have undertaken genome-wide searches for new imprinted genes. These have used empirical approaches, with some success. However, computational prediction of novel imprinted genes has recently come to the forefront. I have developed generalized linear models using data on a variety of sequence and epigenetic features within a training set of known imprinted genes. The resulting models were used to predict novel imprinted genes in the mouse genome. After imposing a stringency threshold, I compiled an initial candidate list of 155 genes. -

Gene Section Review
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL AT INIST-CNRS Gene Section Review PARVB (parvin, beta) Cameron N Johnstone Cancer Metastasis Laboratory, Research Division, Peter MacCallum Cancer Centre, 2 St Andrew's Place, East Melbourne, 3002, Victoria, Australia (CNJ) Published in Atlas Database: April 2010 Online updated version : http://AtlasGeneticsOncology.org/Genes/PARVBID46486ch22q13.html DOI: 10.4267/2042/44936 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 2.0 France Licence. © 2011 Atlas of Genetics and Cytogenetics in Oncology and Haematology Identity DNA/RNA Other names: CGI-56, affixin, beta-parvin Note HGNC (Hugo): PARVB Genethon marker D22S1171 is located at the 5' end of Location: 22q13.31 the gene (Mongroo et al., 2004). Genethon marker Local order: PARVB is located telomeric to the D22S1171 is located between exon 2 and exon 1A of SAMM50 gene and centromeric to the PARVG gene the PARVB gene. at 22q13.31. The PARVA gene is located at 11p15.3. Figure A. Generation of transcript diversity by alternative promoter usage. Horizontal lines above the gene structure indicate human genomic DNA BAC clones. The NCBI accession numbers of the clones, and clone names (in brackets) are shown. Figure adapted from Mongroo et al., 2004. Atlas Genet Cytogenet Oncol Haematol. 2011; 15(1) 34 PARVB (parvin, beta) Johnstone CN Figure B. Human polyA+ RNA Multiple Tissue Northern blot (Origene) probed with full-length PARVB1 cDNA probe radiolabeled to a specific activity of > 5 x 108 cpm / mg (Johnstone C.N., unpublished). The two PARVB mRNA transcripts are indicated. -

Novel Targets of Apparently Idiopathic Male Infertility
International Journal of Molecular Sciences Review Molecular Biology of Spermatogenesis: Novel Targets of Apparently Idiopathic Male Infertility Rossella Cannarella * , Rosita A. Condorelli , Laura M. Mongioì, Sandro La Vignera * and Aldo E. Calogero Department of Clinical and Experimental Medicine, University of Catania, 95123 Catania, Italy; [email protected] (R.A.C.); [email protected] (L.M.M.); [email protected] (A.E.C.) * Correspondence: [email protected] (R.C.); [email protected] (S.L.V.) Received: 8 February 2020; Accepted: 2 March 2020; Published: 3 March 2020 Abstract: Male infertility affects half of infertile couples and, currently, a relevant percentage of cases of male infertility is considered as idiopathic. Although the male contribution to human fertilization has traditionally been restricted to sperm DNA, current evidence suggest that a relevant number of sperm transcripts and proteins are involved in acrosome reactions, sperm-oocyte fusion and, once released into the oocyte, embryo growth and development. The aim of this review is to provide updated and comprehensive insight into the molecular biology of spermatogenesis, including evidence on spermatogenetic failure and underlining the role of the sperm-carried molecular factors involved in oocyte fertilization and embryo growth. This represents the first step in the identification of new possible diagnostic and, possibly, therapeutic markers in the field of apparently idiopathic male infertility. Keywords: spermatogenetic failure; embryo growth; male infertility; spermatogenesis; recurrent pregnancy loss; sperm proteome; DNA fragmentation; sperm transcriptome 1. Introduction Infertility is a widespread condition in industrialized countries, affecting up to 15% of couples of childbearing age [1]. It is defined as the inability to achieve conception after 1–2 years of unprotected sexual intercourse [2]. -

The Genome 10K Project: a Way Forward
The Genome 10K Project: A Way Forward Klaus-Peter Koepfli,1 Benedict Paten,2 the Genome 10K Community of Scientists,Ã and Stephen J. O’Brien1,3 1Theodosius Dobzhansky Center for Genome Bioinformatics, St. Petersburg State University, 199034 St. Petersburg, Russian Federation; email: [email protected] 2Department of Biomolecular Engineering, University of California, Santa Cruz, California 95064 3Oceanographic Center, Nova Southeastern University, Fort Lauderdale, Florida 33004 Annu. Rev. Anim. Biosci. 2015. 3:57–111 Keywords The Annual Review of Animal Biosciences is online mammal, amphibian, reptile, bird, fish, genome at animal.annualreviews.org This article’sdoi: Abstract 10.1146/annurev-animal-090414-014900 The Genome 10K Project was established in 2009 by a consortium of Copyright © 2015 by Annual Reviews. biologists and genome scientists determined to facilitate the sequencing All rights reserved and analysis of the complete genomes of10,000vertebratespecies.Since Access provided by Rockefeller University on 01/10/18. For personal use only. ÃContributing authors and affiliations are listed then the number of selected and initiated species has risen from ∼26 Annu. Rev. Anim. Biosci. 2015.3:57-111. Downloaded from www.annualreviews.org at the end of the article. An unabridged list of G10KCOS is available at the Genome 10K website: to 277 sequenced or ongoing with funding, an approximately tenfold http://genome10k.org. increase in five years. Here we summarize the advances and commit- ments that have occurred by mid-2014 and outline the achievements and present challenges of reaching the 10,000-species goal. We summarize the status of known vertebrate genome projects, recommend standards for pronouncing a genome as sequenced or completed, and provide our present and futurevision of the landscape of Genome 10K.