A Novel Production Method for High-Fructose Glucose Syrup From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sugar: the Many Names Used in Processed Foods
Sugar: the Many Names Used in Processed Foods Both glucose and fructose are common, but they affect the body very differently. Glucose can be metabolized by nearly every cell in the body. Fructose is metabolized almost entirely in the liver. Fructose has harmful effects on the body, including insulin resistance, metabolic syndrome, fatty liver, and type 2 diabetes. It is especially important to minimize the intake of high fructose sugars. Many processed foods will have a combination of sugars. Because the ingredient are listed in order of quantities, using several different sugar names presents the illusion that sugars are less prominent in the ratio of ingredients. Sugar / Sucrose Agave Nectar Sugar with Glucose & Fructose Also knows as table, granulated, or Produced from the agave plant in Various Amounts white sugar, occurring naturally in 79-90% fructose, 10-30% glucose Beet Sugar fruits and plants, added to many Blackstrap Molasses processed foods. Sugar with Fructose Only Brown Sugar, Dark or Light Brown 50% glucose, 50% fructose Crystalline Sugar Fructose Buttered Syrup High Fructose Corn Syrup, HFCS Cane Juice Crystals Sugar without Glucose or HFCS 55 – the most common type Fructose Cane Syrup of HRCS. 55% fructose, 45% D-Ribose, Ribose Cane Sugar glucose, composition is similar to Galactose Caramel sucrose Caramel Color HFCS90 – 90% fructose, 10% Names for Hidden Sugars Carob Syrup glucose Aguamiel Castor Sugar All-natural sweetener Coconut Sugar Names Used to Denote Hight Barbados Molasses Confectioner’s Sugar (Powdered Fructose -

Nutritive Sweeteners from Corn Have Become America’S Premier Sweeteners
NutritiveNutritive SweetenersSweeteners FromFrom CornCorn CONTENTS Member Companies and Plant Locations ....................................... 2 Foreword .......................................................................................... 3 Historical Perspective ...................................................................... 4 Research and development orientation ....................................... 5 Technology aimed at needs .......................................................... 7 Growth, Development and Diversity ............................................. 7 CONTENTS Classification and Nutrition ............................................................ 9 Classification ................................................................................. 9 Corn sweeteners in nutrition ..................................................... 10 Technical Background ................................................................... 11 Corn starch ................................................................................. 11 Starch hydrolysis ........................................................................ 13 Crystalline dextrose .................................................................... 14 Dextrose isomerization .............................................................. 15 Manufacture ................................................................................... 17 Corn syrups ................................................................................ 17 Dried corn syrups ...................................................................... -
Glucose Syrup the Chef’S Magic Ingredient! CENTURIES of GASTRONOMIC TRADITION
Glucose syrup The chef’s magic ingredient! CENTURIES OF GASTRONOMIC TRADITION A product with a proud heritage which plays a significant role in European gastronomy, glucose syrup is used in gourmet foods by pâtissiers, confectioners and chefs alike. This high- quality, plant-based product has been produced in Europe for over a century. In the EU, with its grain-based agriculture, glucose syrup is derived from wheat and maize; EU starch manufacturers source their products exclusively from conventional (non-GMO) crops. Glucose syrup is a sugar made from the hydrolysis (breaking down) of starch. It is available in liquid, solid and transparent form (similar to honey). It was discovered in the 9th century in Japan, and originally derived from sweet potatoes; the glucose syrup manufacturing process was developed in the 19th century by the German scientist, Kirchhoff. A number of culinary specialities benefit from the unique qualities of this ingredient. Bakery products: e.g. pastries, macaroons, cakes etc. Confectionery products: e.g. sweets, lozenges, nougat etc. Glucose syrup plays a vital role in these delicacies... A delight to the eyes and taste buds alike. Still have questions about starch and starch-based ingredients in food? VISIT WWW.STARCHINFOOD.EU TO LEARN MORE. GLUCOSE SYRUP AND DIET EFSA (The European Food Safety Authority)) recommends that carbohydrates should form 45- 60% of our overall energy intake, stating that “enjoyed occasionally and in reasonable quantity, sweetened products are compatible with a balanced diet”. Glucose syrups are part of the simple carbohydrate family, with the same calorific value as all other sugars (sucrose or white sugar, lactose, etc.) i.e. -

Codex Standard for Sugars1 Codex Stan 212-1999 1
CODEX STAN 212-1999 Page 1 of 5 CODEX STANDARD FOR SUGARS1 CODEX STAN 212-1999 1. SCOPE AND DESCRIPTION This Standard applies to the following sugars intended for human consumption without further processing (synonyms are in round brackets). It includes sugars sold directly to the final consumer and sugars used as ingredients in foodstuffs. The description of each of the sugars is also given below: Name Description White sugar Purified and crystallised sucrose (saccharose) with a polarisation not less than 99.7 ºZ. Plantation or mill white sugar Purified and crystallised sucrose (saccharose) with a (or any other equivalent name accepted in the polarisation not less than 99.5 ºZ. country of origin in which it is sold) Powdered sugar Finely pulverised white sugar with or without the addition (icing sugar) of an anticaking agent Soft white sugar Fine grain purified moist sugar, white in colour with a sucrose plus invert sugar content of not less than 97.0% m/m. Soft brown sugar Fine grain purified moist sugar, light to dark brown in colour with a sucrose plus invert sugar content of not less than 88.0% m/m. Dextrose anhydrous Purified and crystallised D-glucose without water of crystallisation, with a D-glucose content of not less than 99.5% m/m on a dry basis and a total solids content of not less than 98.0% m/m. Dextrose monohydrate Purified and crystallised D-glucose containing one molecule of water of crystallisation, with a D-glucose content of not less than 99.5% m/m on a dry basis and a total solids content of not less than 90.0% m/m. -

Sweetright™ Reduced Sugar Glucose Syrup Is Part of Our Sweetright™ Line of Specialty Nutritive, Low and No Calorie Sweeteners
RSGS Sweeten Your Label 67% by Reducing the Sugar are very/ somewhat concerned about It’s simple: you can reduce sugar by replacing your traditional corn syrup sugars & 1 with reduced sugar glucose syrup without changing functionality. sweetners ADM’s reduced sugar glucose syrup helps you reduce sugar by replacing your traditional corn syrup without sacrificing functionality. With lower percentages of mono and disaccharides (DP1 & DP2), reduced sugar 39% glucose syrup means up to 30% less sugar appears on your nutrition indicate reduced sugar is important facts label. ADM’s technical teams can help you easily swap it into your in convenience existing formulations without losing any of the key functional or sensory food, drinks & properties, delivering products consumers will love. snacks2 1,2ADM Outside VoiceSM Sweetening Study SWEETNESS DONE RIGHT SweetRight™ Reduced Sugar Glucose Syrup is part of our SweetRight™ line of specialty nutritive, low and no calorie sweeteners. The SweetRight™ portfolio was created to bring together a comprehensive lineup of offerings that go beyond sweetness – addressing the changing needs for consumer-friendly labels, calorie reduction and health & wellness trends. Unlocking Nature. Enriching Life. 800-257-5743 | [email protected] | adm.com/food RSGS Benefits Beyond Sugar Reduction FUNCTIONAL BENEFITS Reduced sugar glucose syrup offers functional benefits beyond Lowers Added Sugars on label sugar reduction, with unique advantages in target applications. Maintains corn syrup functionality, including bulking & binding FOR A STANDOUT LABEL Provides clean label flexibility Today’s consumers are focused on making better-for-you choices as “Glucose Syrup” or “Corn Syrup” and are on the lookout for labels featuring ‘less added sugars’ and Delivers viscosity comparable to ‘less total sugar’, for themselves and their families. -

Features & Benefits
VERSASWEET™ 1724 Functionality: Low to moderate sweetness, texture Description: Enzyme converted 28 DE glucose syrup with low amounts of mono- and disaccharides and high levels of higher saccharides (DP3+) made from corn Features Benefits Low sugar (DP1/DP2) content Allows for sugar reduction without compromising on texture and taste. Similar processability and viscosity to DE42 glucose Allows for 1:1 replacement of DE42 glucose syrup syrup without changing the processing conditions. Clean label texturising properties Adds creaminess, body and mouthfeel to various food applications. Less hygroscopicity Improves shelf life stability for low moisture products, such as powder creamers and baked goods. Brings less stickiness on final product versus regular glucose syrup VERSASWEET™ 1724 VERSASWEET™ Clean and pleasant sweet taste Improves flavour profile without any off tastes. Low Maillard reactivity Offers stability in hot processes and acidic conditions. APPLICATION AND USAGE INFORMATION Application Summary: VERSASWEET™ 1724 is a glucose syrup with reduced DP1/DP2 content. It offers similar functionality compared to standard 42DE glucose syrup with less added sugars onto the label of the final food application. VERSASWEET™ 1724 enables manufactures to formulate a broad range of sugar reduced products in a variety of categories, including spray dried creamers, beverages and tabletop sauces. Furthermore, VERSASWEET™ 1724 improves the shelf-life stability of various applications including coated snacks, fillings, ice cream and fruit preparations. Typical applications include: Spray dried dairy products: VERSASWEET™ 1724 provides increased shelf-life stability to spray dried coffee creamers. It offers a comparable viscosity to 42DE viscosity, which might improve the spray drying efficiency in comparison to high viscous standard 28DE glucose syrup solids. -

Handbook of Starch Hydrolysis Products and Their Derivatives Haudbook of Starch Hydrolysis Products Aud Their Derivatives
Handbook of Starch Hydrolysis Products and their Derivatives Haudbook of Starch Hydrolysis Products aud their Derivatives Edited by M.W. KEARSLEY Business Development Manager British Sugar Technical Centre Norwich and S.Z. DZIEDZIC Business Development Manager British Sugar Technical Centre Norwich SPRINGER-SCIENCE+BUSINESS MEDIA, B.V. First edition 1995 © 1995 Springer Scicncc+13usincss Media Dordrecht Originally puhlished by Chapman & Hali in 199'1 Softcovcr rcprint ofthe hardcover 1 st cdition 199'1 Typeset in 10/12pt Times by Cambrian Typesetters, Frimley, Surrey ISBN 978-1-4613-5902-9 ISBN 918-1-4615-2159-4 (eBook) DOI 10.1001/918-1-4615-2159-4 Apart from any fair dealing for the purposes of research or private study, or criticism Of review, as permitted under the UK Copyright Designs and Patents Act, 1988, this publication may not be reproduced, stored, or transmitted, in any form or by any means, without thc prior permission in writing of the publishers, or in the case of reprographic reproduction only in accordance with the terms of the licences issued by the Copyright Licensing Agency in the UK, or in accordance with the terms of licences issued by the appropriate Reproduction Rights Organization outside the UK. Enquiries conceming reproduction outside the terms stated here should be sent to the publishers at the Glasgow address printed on this page. The publisher makes no representation, express or implied, with regard to the accuracy of the information contained in this book and cannot accept any legal responsibility or liability for any errors or omissions that may be made. A catalogue record for this book is available from the British Library Library of Congress Catalog Card Number: 95-79038 e Printed on permanent acid-free text paper, manufactured in accordance with ANSIINISO Z39.48-1992 (Permanence of Paper). -

What Is Glucose-Fructose Syrup? (Q&A)
What is Glucose-Fructose Syrup? (Q&A) 05 April 2018 Glucose-fructose syrup is a sweetening ingredient widely used in a variety of food products. Read our Q&As for more information about it, how it is made and its impact on our health. What are glucose and fructose? Glucose is a simple sugar, a so-called monosaccharide, because it is made up of just one sugar unit. It is found naturally in many foods, and it is used by our bodies as a source of energy to carry out daily activities. Fructose is also a simple sugar, often referred to as a fruit sugar. Fructose, as the name suggest, is found in fruits (such as oranges and apples), berries, some root vegetables (such as beets, sweet potatoes, parsnips, and onions) and honey. Fructose is the sweetest of all naturally occurring sugars. Glucose and fructose bound together in equal amounts create another type of sugar – sucrose – a disaccharide commonly known as table sugar. What is Glucose-Fructose Syrup (GFS)? GFS is a sweet liquid made of glucose and fructose. Unlike sucrose, where 50% of glucose and 50% of fructose are linked together, GFS can have a varying ratio of the two simple sugars, meaning that some extra, unbound glucose or fructose molecules are present. The fructose content in GFS can range from 5% to over 50%. How is GFS made? GFS is typically made from starch. The source of starch depends on the local availability of the raw product used for extraction. Historically, maize was a preferred choice, while in recent years wheat became a popular source for the GFS production. -

Label Reading
Eliminating FODMAPs Label Reading Reading labels is one of the trickiest parts of the low FODMAP diet! I recommend that you limit processed foods during the elimination phase but it is impossible to avoid packaged foods completely. You have to become an excellent detective at spotting the FODMAPs as well as other possible gut irritants. They sometimes sneak in where you least expect it. On food labels, keep in mind that ingredients are listed in order of weight. This means the first ingredient is in the largest quantity and the last ingredient is in the lowest quantity. If you are unsure about a product, you can phone the food manufacturer. The follow list is based on the most current information available and is subject to change as more foods are analyzed. Please note that some of the foods have not yet been tested for FODMAPs. If you have questions, you can email me a photos of the label and I will have a look. Audrey Inouy e, Registered Dietitian www.ibsnutrition.ca Page 1 Eliminating FODMAPs Low FODMAP ingredients: Asafoetida Icing sugar Aspartame Invert sugar Baker's yeast Locust bean gum Baking powder Malt extract Baking soda Maltodextrin Barley malt Maltose Beet sugar Maple syrup Berry sugar Modified food starch Black pepper Brown sugar Palm sugar Brown sugar Pectin Buckwheat flour Raw sugar Cane juice crystals refined sugar Cane sugar Resistant starch Castor sugar Rice malt Cellulose Rice protein Cocoa powder <3tsp Saccharine Corn syrup and corn syrup solids* Soy lecithin Cultured corn syrup Soybean oil Dehydrated sugar and juice Soy protein isolate Dextrose soy sauce Egg Protein Stevia Erythritol Sucrose Glucose Wheat dextrin Glucose syrup Wheat maltodextrin* Golden syrup Wheat starch Granulate sugar Whey protein isolate Guar gum White sugar High-maltose corn syrup Xanthan Gum Highlighted foods are low FODMAP but can be fermented in our gut which means that some people with IBS may have additional issues with them not related to FODMAPs. -

Glucose Syrup 80% F9 Product Description
Glucose Syrup 80% F9 Product Description Glucose syrup 80 % DE 40 is an aqueous solution of saccharides containing glucose, maltose and oligosaccharides obtained from wheat and/or corn starch by enzymatic hydrolysis. It is suitable for foodstuffs and is intended for human consumption. Specification Description Nutritional Values per 100 g clear, colourless to Nutritional Value 1.334 kJ/ 314 kcal Appearance slightly yellowish syrup Fat 0,0 g Taste pure, sweet - of which saturated 0,0 g Odour neutral, typical Carbohydrates 78,5 g wheat and/or corn Raw Material - of which sugar 52,6 g starch Fibres 0,0 g Protein 0,0 g Salt 0,0 g Physical-Chemical Analysis Minimum Maximum Analysis Method Dry Substance (%) 77,5 81,5 CRA-RIDS conversion table Brix (20°C) 78,4 82,0 Density Meter (flexural resonator) RI (20°C) 1,493 1,496 Refractometer pH 3,0 5,5 pH-Meter Dextrose Equivalent (% DS) 56,0 68,0 Lane Eynon titration SO2 [mg/kg] 10 Titration Sugar Composition (% DS) Minimum Maximum Analysis Method Fructose 8,0 10,0 HPLC Glucose 22,0 35,0 HPLC Maltose 29,0 43,0 HPLC Trisaccharides 5,0 14,0 HPLC Higher Sugars 8,0 32,0 HPLC Microbiology (CFU/g) Total Plate Count max. 200 Yeasts max. 10 Moulds max. 10 Eurosweet GmbH / Bruktererstr. 10 / 46238 Bottrop, Germany / Managing Director: Robert Rutherford Commercial Register: Gelsenkirchen HRB 7919 Member of the Galam Group Page 1 of 2 Standard Packaging - Container from 200 bis 1.350 kg - Bulk tank truck up to 25 t Storage Protect from environmental influences and strong changes of temperature. -
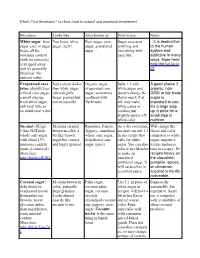
What's That Sweetener? (A Cheat Sheet to Natural and Unnatural Sweeteners)
What's That Sweetener? (a cheat sheet to natural and unnatural sweeteners) Sweetener Looks like Also known as How to use Notes White sugar: from You know white Beet sugar, cane Sugar sweetens ...it is destructive sugar cane or sugar sugar, right? sugar, granulated anything and to the human beets; all the sugar everything with system and molasses content ease, but... addictive in many (with its minerals) ways. More here: is stripped away http://bit.ly/UquV and it's generally Cf bleached. No nutrient value. Evaporated cane Just a shade darker Organic sugar, Subs 1:1 with A good choice if juice: slightly less than white sugar, evaporated cane white sugar and organic, non- refined cane sugar; often slightly sugar, sometimes doesn't change the GMO or fair trade a small step up larger grained but confused with flavor much if at sugar is from white sugar; not necessarily Turbinado all; may make important to you. still very little to white cakes or It's a large step no nutritional value cookies just up in price for a slightly darker off- small step in white color nutrition. Sucanat (SUgar Medium caramel Rapadura, Panela, As a dry sweetener, Will change the CAne NATural): brown in color, a Jaggery, unrefined sucanat can sub 1:1 flavor and color whole cane sugar bit like brown whole cane sugar, in any recipe that compared to white with about 13% sugar but coarser dehydrated cane calls for white sugar; imparts a molasses content and larger grained. sugar (juice) sugar. You can also deeper molasses (none is removed). -

Sugar: How Sweet It Is
1 Copyright © 2014 The Health Coach Group, Inc. All Right Reserved. No part of this book may be reproduced or redistributed in any form or by any electronic or mechanical means, including information storage and retrieval systems, without permission in writing from the publisher. Published in the United States by: The Health Coach Group, Inc. 7601 Military Avenue, Omaha, NE 68134 http://www.thehealthcoachgroup.com. The information contained in this book is intended to help readers make informed decisions about their health. It should not be used as a substitute for treatment by or the advice of a physician. Although the author and publisher have worked hard to ensure that the information provided here is complete and accurate, they will not be held responsible for loss or damage of any nature suffered as a result of reliance on any of this book’s contents or any errors or omissions herein. Copyright 2014 2 The Health Coach Group FACT: • Sugar is 8 x’s more addictive than cocaine. • Sugar cause obesity, inflammation (disease), feeds cancer, dementia, type 2 diabetes, depression, infertility, impotence, depression and brain function. “The average American consumes about 152 pounds of sugar a year. That’s roughly 22 teaspoons every day for every person in America. And our kids consume about 34 teaspoons every day — that’s more than two 20-ounce sodas — making nearly one in four teenagers pre-diabetic or diabetic. Flour is even worse than sugar. We consume about 146 pounds of flour a year. Think about it. That’s about one pound of sugar and flour combined every day for every man, woman and child in America.