Academic Year 2018- 2019 Third Term Chemistry Revision Sheets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Comparison of Sulfur to Oxygen*
OpenStax-CNX module: m34977 1 Comparison of Sulfur to Oxygen* Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 1 Size Table 1 summarizes the comparative sizes of oxygen and sulfur. Element Atomic radius Covalent radius Ionic radius (Å) van der Waal ra- (Å) (Å) dius (Å) Oxygen 0.48 0.66 1.40 1.52 Sulfur 0.88 1.05 1.84 1.80 Table 1: Comparison of physical characteristics for oxygen and sulfur. 2 Electronegativity Sulfur is less electronegative than oxygen (2.4 and 3.5, respectively) and as a consequence bonds to sulfur are less polar than the corresponding bonds to oxygen. One signicant result in that with a less polar S-H bond the subsequent hydrogen bonding is weaker than observed with O-H analogs. A further consequence of the lower electronegativity is that the S-O bond is polar. 3 Bonds formed Sulfur forms a range of bonding types. As with oxygen the -2 oxidation state prevalent. For example, sulfur forms analogs of ethers, i.e., thioethers R-S-R. However, unlike oxygen, sulfur can form more than two covalent (non-dative) bonds, i.e., in compounds such as SF4 and SF6. Such hypervalent compounds were originally thought be due to the inclusion of low energy d orbitals 3 2 in hybrids (e.g., sp d for SF6); however, a better picture involves a combination of s and p ortbitals in bonding (Figure 1). Any involvement of the d orbitals is limited to the polarization of the p orbitals rather than direct hydridization. -
Effect of Catenation and Basicity of Pillared Ligand on the Water Stability of Mofs
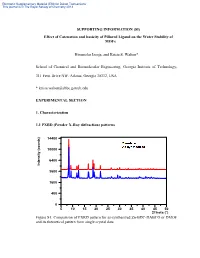
Electronic Supplementary Material (ESI) for Dalton Transactions This journal is © The Royal Society of Chemistry 2013 SUPPORTING INFORMATION (SI) Effect of Catenation and basicity of Pillared Ligand on the Water Stability of MOFs Himanshu Jasuja, and Krista S. Walton* School of Chemical and Biomolecular Engineering, Georgia Institute of Technology, 311 Ferst Drive NW, Atlanta, Georgia 30332, USA * [email protected] EXPERIMENTAL SECTION 1. Characterization 1.1 PXRD (Powder X-Ray diffraction) patterns 14400 10000 6400 Intensity (counts) Intensity 3600 1600 400 0 5 10 15 20 25 30 35 40 45 50 2Theta (°) Figure S1: Comparison of PXRD pattern for as-synthesized Zn-BDC-DABCO or DMOF and its theoretical pattern from single crystal data Electronic Supplementary Material (ESI) for Dalton Transactions This journal is © The Royal Society of Chemistry 2013 14400 10000 6400 Intensity (counts) Intensity 3600 1600 400 0 5 10 15 20 25 30 35 40 45 50 2Theta (°) Figure S2: Comparison of PXRD pattern for as-synthesized Zn-BDC-BPY or MOF-508a and its theoretical pattern from single crystal data 14400 10000 6400 Intensity (counts) Intensity 3600 1600 400 0 5 10 15 20 25 30 35 40 45 50 2Theta (°) Figure S3: Comparison of PXRD pattern for as-synthesized Zn-TMBDC-DABCO or DMOF-TM and its theoretical pattern from single crystal data Electronic Supplementary Material (ESI) for Dalton Transactions This journal is © The Royal Society of Chemistry 2013 14400 10000 6400 Intensity (counts) Intensity 3600 1600 400 0 5 10 15 20 25 30 35 40 45 50 2Theta (°) Figure S4: Comparison of PXRD pattern for as-synthesized Zn-TMBDC-BPY or MOF- 508-TM and its theoretical pattern from single crystal data 6400 3600 Intensity (counts) Intensity 1600 400 0 10 15 20 25 30 35 40 45 2Theta (°) Figure S5: PXRD patterns for as-synthesized Zn-BDC-BPY or MOF-508a and activated Zn-BDC-BPY or MOF-508b displaying shifting of peaks towards right on activation which was also observed by Chen et. -

United States Patent C Patented Aug
3,336,412 United States Patent C Patented Aug. 15, 1967 1 2 3,336,412 tion reaction can be eliminated by the addition of a halogen PRODUCTION OF UNSATURATED HYDROCAR gas to the reaction mixture. In one preferred embodiment BGNS BY PYROLYSIS 0F SATURATED HY it has now been discovered that the addition of chlorine DROCARBONS to a mixture of methane and oxygen increases the yield of Richard Kenneth Lyon, Elizabeth, and William Bartok, acetylene and eliminates the need for preheating‘ the West?eld, N.J., assignors to Esso Research and Engi neering Company, a corporation of Delaware methane and oxygen reactants. While not wishing to be No Drawing. Filed June 29, 1964, Ser. No. 379,031 bound by any particular theory, it is believed that the ad_ 8 Claims. (Cl. 260-679) dition of chlorine promotes the formation of acetylene in the reaction 3H2+C2<:>2CH4 by formation of HCl This invention relates ‘to an improved process for the 10 thereby driving the reaction in accordance with familiar pyrolysis of certain saturated hydrocarbons to obtain un principles of equilibrium reaction. Furthermore, the re saturated hydrocarbons. More particularly, this invention action H2+Cl2—>2HCl is exothermic and therefore adds relates to the production of unsaturated hydrocarbons additional heat to the reaction. The utilization of chlorine by partial combustion of saturated hydrocarbons. In a pre gas with the unsaturated hydrocarbon-oxygen mixture ferred embodiment, this invention relates to the produc 15 possesses a further advantage in that the halogen gas will tion of acetylene by partial combustion of hydrocarbons not react with the unsaturated hydrocarbon as will water, such as methane. -

Studies of the Temporary Anion States of Unsaturated Hydrocarbons by Electron Transmission Spectroscopy
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Paul Burrow Publications Research Papers in Physics and Astronomy 1978 Studies of the Temporary Anion States of Unsaturated Hydrocarbons by Electron Transmission Spectroscopy Kenneth D. Jordan Paul Burrow Follow this and additional works at: https://digitalcommons.unl.edu/physicsburrow Part of the Atomic, Molecular and Optical Physics Commons This Article is brought to you for free and open access by the Research Papers in Physics and Astronomy at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Paul Burrow Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. digitalcommons.unl.edu Studies of the Temporary Anion States of Unsaturated Hydrocarbons by Electron Transmission Spectroscopy Kenneth D. Jordan Mason Laboratory, Department of Engineering and Applied Science, Yale University, New Haven, Connecticut 06520 Paul D. Burrow Behlen Laboratory of Physics, University of Nebraska, Lincoln, Nebraska 68588 The concept of occupied and unoccupied orbitals has provided a useful means for visualizing many of the most important properties of mo- lecular systems. Yet, there is a curious imbalance in our experimen- tal knowledge of the energies of occupied and unoccupied orbitals. Whereas photoelectron spectroscopy has provided a wealth of data on positive ion states and has established that they can be associated, within the context of Koopmans’ theorem, with the occupied orbitals of the neutral molecule, the corresponding information for the neg- ative ion states, associated with the normally unoccupied orbitals, is sparse. In part this reflects the experimental difficulties connected with measuring the electron affinities of molecules which possess sta- ble anions. -

Chemistry Study Materials for Class 10 (NCERT Based: Questions with Answers) Ganesh Kumar Date:- 28/07/2020
Chemistry Study Materials for Class 10 (NCERT Based: Questions with Answers) Ganesh Kumar Date:- 28/07/2020 74. How much carbon is present on earth and CO2 in atmosphere? Answer: 0.02%, 0.03% 75. What is valency of carbon? Answer: 4 76. Why does carbon form strong bonds? Answer: It is due to small size. 77. What will be the product formed when carbon is burnt in presence of air? Answer: Carbon dioxide 78. What is Allotropy? Answer: It is a property due to which an element can exist in more than one form which differ in physical properties but have similar chemical properties, e.g., carbon, sulphur, phosphorus, oxygen show allotropy. 79. Name three allotropes of carbon. Answer: Diamond, graphite and Buckminster fullerenes 80. Which is the purest allotrope of carbon? Answer: Buckminster fullerenes. 81. Why is graphite soft and slippery? Answer: Due to weak vander Waals’ forces of attraction between hexagonal layers. 82. Why is diamond hard? Answer: It is due to strong covalent bonds. 83. Why is diamond lustrous? Answer: It is due to high refractive index. 84. Carbon has four electrons in its valence shell. How does carbon attain stable electronic configuration. Answer: By sharing four electrons with other atoms. 85. Why is carbon tetravalent? Answer: It is because carbon can share form electrons to complete its octet. 86. Which gas is present in LPG? Answer: Butane and Isobutane 87. Which element exhibits the property of catenation to maximum extent and why? Answer: Carbon shows catenation to maximum extent because it forms strong covalent bonds. -

MODULE 11: GLOSSARY and CONVERSIONS Cell Engines
Hydrogen Fuel MODULE 11: GLOSSARY AND CONVERSIONS Cell Engines CONTENTS 11.1 GLOSSARY.......................................................................................................... 11-1 11.2 MEASUREMENT SYSTEMS .................................................................................. 11-31 11.3 CONVERSION TABLE .......................................................................................... 11-33 Hydrogen Fuel Cell Engines and Related Technologies: Rev 0, December 2001 Hydrogen Fuel MODULE 11: GLOSSARY AND CONVERSIONS Cell Engines OBJECTIVES This module is for reference only. Hydrogen Fuel Cell Engines and Related Technologies: Rev 0, December 2001 PAGE 11-1 Hydrogen Fuel Cell Engines MODULE 11: GLOSSARY AND CONVERSIONS 11.1 Glossary This glossary covers words, phrases, and acronyms that are used with fuel cell engines and hydrogen fueled vehicles. Some words may have different meanings when used in other contexts. There are variations in the use of periods and capitalization for abbrevia- tions, acronyms and standard measures. The terms in this glossary are pre- sented without periods. ABNORMAL COMBUSTION – Combustion in which knock, pre-ignition, run- on or surface ignition occurs; combustion that does not proceed in the nor- mal way (where the flame front is initiated by the spark and proceeds throughout the combustion chamber smoothly and without detonation). ABSOLUTE PRESSURE – Pressure shown on the pressure gauge plus at- mospheric pressure (psia). At sea level atmospheric pressure is 14.7 psia. Use absolute pressure in compressor calculations and when using the ideal gas law. See also psi and psig. ABSOLUTE TEMPERATURE – Temperature scale with absolute zero as the zero of the scale. In standard, the absolute temperature is the temperature in ºF plus 460, or in metric it is the temperature in ºC plus 273. Absolute zero is referred to as Rankine or r, and in metric as Kelvin or K. -

Group 13 Elements
GROUP I5 ELEMENTS Q. 1. Give chemical reaction in support of the statement that all the bonds in PCI5 molecule are not equivalent. Ans. Due to greater bond pair-bond pair repulsions, the two axial P — Cl bonds are less stable than the three equatorial P — Cl bonds. It is because of this reason that when PC15 is heated, the less stable axial bonds are Broken to form Cl2. Δ PCl5 -------------- PCl3 + Cl2. Q. 2. The tendency for catenation decreases down the group 14. Or Why carbon shows catenation but silicon does not Ans. As we move down the group 14, the atomic size increases and hence the strength of M—M bond decreases steadily. Consequently the tendency for catenation decreases down the group. Q. 3. PCI5 is known but NC15 is not known. Explain. 2 2 6 2 1 1 1 Ans. Electronic configuration of P is 1s 2s 2p 3s 3px 3py 3pz . Thus phosphorus has empty 3d orbitals in which the elecron of the 3s-orbital can be excited but nitrogen has no d-orbitals as its electronic configuration is 2 2 1 1 1 1s 2s 2px 2py 2pz . Q. 4. Why molecular nitrogen is not reactive ? Ans. The bond dissociation energy for the triple bond in N = N is very high because of small size of N-atoms and hence small internuclear distance. Q.5. H3PO3 is diprotic (or)dibasic. Why? Ans. Since it has only two ionisable H – atoms which are present as OH groups. Q. 6. Nitrogen exists as diatomic molecule, N2, whereas phosphorus exists a tetratomic molecule P4. -

Unit 12 Carbon Akd Its Compounds
UNIT 12 CARBON AKD ITS COMPOUNDS Structure 12.1 Introduction 12.2 Objectives 12.3 Allotropes of Carbon 12.4 Why are there a Large Number of Carbon Compounds in Nature 12.4.1 Catenation 12.4.2 Isomerism 12.5 Hydrocarbons 12.5.1 Saturated Hydrocarbons 12.5.2 Unsaturated Hydrocarbons 12.5.3 Homologous Hydrocarbons 12.5.4 Aromatic Hydrocarbons 12.6 Some other Organic Compounds 12.6.1 Hydrocarbon as Parent Compound 12.6.2 Functional Groups 12.6.3 Alcohols 12.6.4 Carboxylic Acids 12.6.5 Esters 12.7 Man Made Materials from Carbon Compounds 12.7.1 Polymers-Fibres, Plastics, Rubbers 12.7.2 Soaps and Detergents 12.8 Let Us Sum Up 12.9 Unit-end Exercises 12.10 Answers to Check Your Progress 12.11 Suggested Readings 12.1 INTRODUCTION In the previous unit you have studied about the strategies of teaching about some metallic and non-metallic elements and some of their compounds. In the present unit we shall discuss the teaching of some exemplary concepts and subtopics related to carbon and its compounds. Here it is important to appreciate that carbon and organic compounds need to be dealt as separate from other elements and their compounds. As you go through this unit you would be familiarizing yourself with teaching of allotropic forms of carbon, hydrocarbons and other compounds of carbon and finally the man made materials obtained from carbon compounds. This unit shows us that living bodies and their products contain a large quantity of carbon compounds in our daily life. -

25WORDS ETHYLENE Ethylene, C2H4 ,Is an Unsaturated
25WORDS ETHYLENE Ethylene, C2H4 ,is an unsaturated hydrocarbon that is used in industrial plants and sometimes as a hormone in an average medicine cabinet. It also is the most globally produced organic compound in the world. Ethylene, C2H4, is a hydrocarbon gas that is widely used in the world's industry for purposes like ripening fruit, making detergents, and for making soda. It is also highly flammable and colorless. Ethylene is an unsaturated hydrocarbon, composed of four hydrogen atoms bound to a pair of carbon atoms by means of a double bond. Ethylene has a molar mass of 28.05 g/mol Ethylene is the simplest member of the class called alkenes. It is a colorless, quite sweet- smelling gas. This gas is very reactive and burns with a very bright flame. ethylene (C2H4); Ethylene is known as the simplest alkene and an important hormone in organic chemistry. Over 80% of ethylene is used as a main component of polyethylene and to ripen fruit faster. Ethylene, C2H4, is a colorless gas that can be used as an inhalation anesthetic. This gas is also commonly used to keep fruit ripe as well as to cut and wield metals. Ethylene, C2H4, is an unsaturated hydrocarbon. It is used in anesthetic agents and in detergents. It is the most widely produced organic compound in the world. Ethylene (C2H4): Ethylene is an unsaturated hydrocarbon that is used in the production of polyethylene, a widely used plastic. It can be modified to become ethylene glycol (an antifreeze) and ethylene dichloride (used in creating polyvinyl chloride Ethlyene; Ethylene, C2H4, is a colorless, odorless gas that can be produced in nature as well as man-made processes. -

WO 2015/000840 Al 8 January 2015 (08.01.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/000840 Al 8 January 2015 (08.01.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every CIOG 67/04 (2006.01) CIOG 69/06 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/EP2014/063848 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 30 June 2014 (30.06.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13 174781 .8 2 July 2013 (02.07.2013) EP (84) Designated States (unless otherwise indicated, for every (71) Applicants: SAUDI BASIC INDUSTRIES CORPORA¬ kind of regional protection available): ARIPO (BW, GH, TION [SA/SA]; P.O. Box 5101, 11422 Riyadh (SA). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, SABIC GLOBAL TECHNOLOGIES [NL/NL]; Plastics- UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, laan 1, NL-4612 PX Bergen Op Zoom (NL). -

Chapter 13 "Unsaturated and Aromatic Hydrocarbons"
This is “Unsaturated and Aromatic Hydrocarbons”, chapter 13 from the book Introduction to Chemistry: General, Organic, and Biological (index.html) (v. 1.0). This book is licensed under a Creative Commons by-nc-sa 3.0 (http://creativecommons.org/licenses/by-nc-sa/ 3.0/) license. See the license for more details, but that basically means you can share this book as long as you credit the author (but see below), don't make money from it, and do make it available to everyone else under the same terms. This content was accessible as of December 29, 2012, and it was downloaded then by Andy Schmitz (http://lardbucket.org) in an effort to preserve the availability of this book. Normally, the author and publisher would be credited here. However, the publisher has asked for the customary Creative Commons attribution to the original publisher, authors, title, and book URI to be removed. Additionally, per the publisher's request, their name has been removed in some passages. More information is available on this project's attribution page (http://2012books.lardbucket.org/attribution.html?utm_source=header). For more information on the source of this book, or why it is available for free, please see the project's home page (http://2012books.lardbucket.org/). You can browse or download additional books there. i Chapter 13 Unsaturated and Aromatic Hydrocarbons Opening Essay Our modern society is based to a large degree on the chemicals we discuss in this chapter. Most are made from petroleum. In Chapter 12 "Organic Chemistry: Alkanes and Halogenated Hydrocarbons" we noted that alkanes—saturated hydrocarbons—have relatively few important chemical properties other than that they undergo combustion and react with halogens. -

2. Allotropy in Carbon the Property Due to Which an Element Exists In
SUBJECT-CHEMISTRY DATE- 22/11/2020 CLASS- X TOPICS- Chap. 4 Carbon and its Compounds Carbon is one of the most essential components of living organisms. There are two stable isotopes of carbon C-12 and C-13. After these two one more isotope of carbon is present C-14. Carbon is used for radiocarbon dating One of the most amazing properties of carbon is its ability to make long carbon chains and rings. This property of carbon is known as catenation. Carbon has many special abilities out of all one unique ability is that carbon forms double or triple bonds with itself and with other electronegative atoms like oxygen and nitrogen. These two properties of carbon i.e catenation and multiple bond formation, it has the number of allotropic forms. Allotrope is nothing but the existence of an element in many forms which will have different physical property but will have similar chemical properties and its forms are called allotropes of allotropic forms. Allotropes are defined as the two or more physical forms of one element. These allotropes are all based on carbon atoms but exhibit different physical properties, especially with regard to hardness. The two common, crystalline allotropes of carbon are diamond and graphite. Carbon shows allotropy because it exists in different forms of carbon. Though these allotropes of carbon have a different crystal structure and different physical properties, their chemical properties are the same and show similar chemical properties. Both diamond and graphite have symbol C. Both give off carbon dioxide when strongly heated in the presence of oxygen.