Evaluation of Anti Depressant Activity of Calotropis Gigantea by Using
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Taxonomic Revision of Rhododendron L. Section Pentanthera G
A TAXONOMIC REVISION OF RHODODENDRON L. SECTION PENTANTHERA G. DON (ERICACEAE) BY KATHLEEN ANNE KRON A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 1987 , ACKNOWLEDGMENTS I gratefully acknowledge the supervision and encouragement given to me by Dr. Walter S. Judd. I thoroughly enjoyed my work under his direction. I would also like to thank the members of my advisory committee, Dr. Bijan Dehgan, Dr. Dana G. Griffin, III, Dr. James W. Kimbrough, Dr. Jonathon Reiskind, Dr. William Louis Stern, and Dr. Norris H. Williams for their critical comments and suggestions. The National Science Foundation generously supported this project in the form of a Doctoral Dissertation Improvement Grant;* field work in 1985 was supported by a grant from the Highlands Biological Station, Highlands, North Carolina. I thank the curators of the following herbaria for the loan of their material: A, AUA, BHA, DUKE, E, FSU, GA, GH, ISTE, JEPS , KW, KY, LAF, LE NCSC, NCU, NLU NO, OSC, PE, PH, LSU , M, MAK, MOAR, NA, , RSA/POM, SMU, SZ, TENN, TEX, TI, UARK, UC, UNA, USF, VDB, VPI, W, WA, WVA. My appreciation also is offered to the illustrators, Gerald Masters, Elizabeth Hall, Rosa Lee, Lisa Modola, and Virginia Tomat. I thank Dr. R. Howard * BSR-8601236 ii Berg for the scanning electron micrographs. Mr. Bart Schutzman graciously made available his computer program to plot the results of the principal components analyses. The herbarium staff, especially Mr. Kent D. Perkins, was always helpful and their service is greatly appreciated. -

The Red List of Rhododendrons
The Red List of Rhododendrons Douglas Gibbs, David Chamberlain and George Argent BOTANIC GARDENS CONSERVATION INTERNATIONAL (BGCI) is a membership organization linking botanic gardens in over 100 countries in a shared commitment to biodiversity conservation, sustainable use and environmental education. BGCI aims to mobilize botanic gardens and work with partners to secure plant diversity for the well-being of people and the planet. BGCI provides the Secretariat for the IUCN/SSC Global Tree Specialist Group. Published by Botanic Gardens Conservation FAUNA & FLORA INTERNATIONAL (FFI) , founded in 1903 and the International, Richmond, UK world’s oldest international conservation organization, acts to conserve © 2011 Botanic Gardens Conservation International threatened species and ecosystems worldwide, choosing solutions that are sustainable, are based on sound science and take account of ISBN: 978-1-905164-35-6 human needs. Reproduction of any part of the publication for educational, conservation and other non-profit purposes is authorized without prior permission from the copyright holder, provided that the source is fully acknowledged. Reproduction for resale or other commercial purposes is prohibited without prior written permission from the copyright holder. THE GLOBAL TREES CAMPAIGN is undertaken through a partnership between FFI and BGCI, working with a wide range of other The designation of geographical entities in this document and the presentation of the material do not organizations around the world, to save the world’s most threatened trees imply any expression on the part of the authors and the habitats in which they grow through the provision of information, or Botanic Gardens Conservation International delivery of conservation action and support for sustainable use. -

Rhododendron
DOI:10.2478/JAS-2020-0028 J. APIC. SCI. VOL. 64 NO. 2 2020J. APIC. SCI. Vol. 64 No. 2 2020 Original Article PROPERTIES OF HONEY AND POLLEN SAMPLES OBTAINED FROM DIFFERENT RHODODENDRON SPECIES COLLECTED FROM BLACK SEA REGION OF TURKEY Sezai Alkan1* Mert Akgün1 Ömer Ertürk2 Melek Çol Ayvaz1 Ceren Başkan3 1Department of Animal Science, Faculty of Agriculture, Ordu University, Ordu, Turkey 2Department of Molecular Biology and Genetics, Faculty of Science and Arts, Ordu University, Ordu, Turkey 3Sabuncuoğlu Şerefeddin Health Services Vocational School, Amasya University, Amasya, Turkey *corresponding author: [email protected] Received: 04 March 2020; accepted: 26 July 2020 Abstract Physicochemical properties as well as antioxidant and antimicrobial capabilities of Rhododendron honey and pollen produced in Turkey were determined. Monofloral honey samples from three different Rhododendron species (R. ponticum L., R. luteum L., and R. caucasicum L.) were collected from the mountains of the Eastern Black Sea Region of Turkey. The experimental results revealed that each crude extract of honey and pollen exhibited significant antibacterial and antifungal capacity in the bacteria and fungus. The pollen samples and SEM images have been analysed and recorded. The total phenolic contents and antioxidative activities of the samples were investigated based on DPPH free radical scavenging activities and ferric reducing antioxidative power potentials, and higher phenolic content and antioxidant activities were observed for pollen samples with respect to honey. Furthermore, the potential to inhibit Acetyl- and Butrylcholinesterase activity and lipid peroxidation were evaluated. The potential to inhibit DNA damage were also studied, and R. ponticum honey was observed to influence most positively damaged DNA. -

Biological Activities and Cytotoxicity of Leaf Extracts from Plants of the Genus Rhododendron
From Ethnomedicine to Application: Biological Activities and Cytotoxicity of Leaf Extracts from Plants of the Genus Rhododendron by Ahmed Rezk a Thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biochemistry Approved Dissertation Committee Prof. Dr. Matthias Ullrich, Prof. of Microbiology Prof. Dr. Klaudia Brix, Prof. of Cell Biology Jacobs University Bremen Prof. Dr. Nikolai Kuhnert Prof. of Chemistry Jacobs University Bremen Prof. Dr. Dirk Albach, Prof. of Plant Biodiversity University of Oldenburg Date of Defense: 15.06.2015 This PhD thesis project was financed by Stiftung Rhododendronpark Bremen Dedicated to: My Wife Rasha Acknowledgment Acknowledgment First, I thank Allah for giving me the ability and strength to accomplish this study. I would like to express my gratitude to the following people for support during my work: I would like to express my sincere appreciation and gratitude to my PhD supervisors, Prof. Dr. Matthias Ullrich, and Prof. Dr. Klaudia Brix, who gave me the opportunity to compose my doctoral thesis in their workgroups. I would like to thank them for their support, guidance and all the time they gave to discuss and help in designing experiments to achieve this work. I would also like to thank my dissertation committee members, Prof. Dr. Nikolai Kuhnert and Prof. Dr. Dirk Albach for their time and for their valuable comments during our meetings and reviewing my thesis. I would specifically like to thank AG Ullrich and AG Brix lab members, Amna Mehmood, Antje Stahl, Gabriela Alfaro-Espinoza, Khaled Abdallah, Neha Kumari, Maria Qatato, Joanna Szumska, and Jonas Weber for maintaining a friendly and family working environment. -

Plant Species and Communities in Poyang Lake, the Largest Freshwater Lake in China
Collectanea Botanica 34: e004 enero-diciembre 2015 ISSN-L: 0010-0730 http://dx.doi.org/10.3989/collectbot.2015.v34.004 Plant species and communities in Poyang Lake, the largest freshwater lake in China H.-F. WANG (王华锋)1, M.-X. REN (任明迅)2, J. LÓPEZ-PUJOL3, C. ROSS FRIEDMAN4, L. H. FRASER4 & G.-X. HUANG (黄国鲜)1 1 Key Laboratory of Protection and Development Utilization of Tropical Crop Germplasm Resource, Ministry of Education, College of Horticulture and Landscape Agriculture, Hainan University, CN-570228 Haikou, China 2 College of Horticulture and Landscape Architecture, Hainan University, CN-570228 Haikou, China 3 Botanic Institute of Barcelona (IBB-CSIC-ICUB), pg. del Migdia s/n, ES-08038 Barcelona, Spain 4 Department of Biological Sciences, Thompson Rivers University, 900 McGill Road, CA-V2C 0C8 Kamloops, British Columbia, Canada Author for correspondence: H.-F. Wang ([email protected]) Editor: J. J. Aldasoro Received 13 July 2012; accepted 29 December 2014 Abstract PLANT SPECIES AND COMMUNITIES IN POYANG LAKE, THE LARGEST FRESHWATER LAKE IN CHINA.— Studying plant species richness and composition of a wetland is essential when estimating its ecological importance and ecosystem services, especially if a particular wetland is subjected to human disturbances. Poyang Lake, located in the middle reaches of Yangtze River (central China), constitutes the largest freshwater lake of the country. It harbours high biodiversity and provides important habitat for local wildlife. A dam that will maintain the water capacity in Poyang Lake is currently being planned. However, the local biodiversity and the likely effects of this dam on the biodiversity (especially on the endemic and rare plants) have not been thoroughly examined. -

Mendelova Univerzita V Brně
MENDELOVA UNIVERZITA V BRNĚ ZAHRADNICKÁ FAKULTA V LEDNICI Použití rodu Rhododendron L. v zahradní a krajinářské architektuře za první republiky Diplomová práce Vypracoval: Bc. Tomáš Schuch Vedoucí práce: prof. Ing. Miloš Pejchal, CSc. Lednice, 2015 Čestné prohlášení Prohlašuji, že jsem diplomovou práci na téma „Použití rodu Rhododendron L. v zahradní a krajinářské architektuře za první republiky“ vypracoval samostatně a veškeré použité prameny a informace uvádím v seznamu použité literatury. Souhlasím, aby moje práce byla zveřejněna v souladu s § 47b zákona č. 111/1998 Sb., o vysokých školách ve znění pozdějších předpisů a v souladu s platnou Směrnicí o zveřejňování vysokoškolských závěrečných prací. Jsem si vědom, že se na moji práci vztahuje zákon č. 121/2000 Sb., autorský zákon, a že Mendelova univerzita v Brně má právo na uzavření licenční smlouvy a užití této práce jako školního díla podle § 60 odst. 1 autorského zákona. Dále se zavazuji, že před sepsáním licenční smlouvy o využití díla jinou osobou (subjektem) si vyžádám písemné stanovisko univerzity, že předmětná licenční smlouva není v rozporu s oprávněnými zájmy univerzity, a zavazuji se uhradit případný příspěvek na úhradu nákladů spojených se vznikem díla, a to až do jejich skutečné výše. V Lednici dne: …………………………………………….. podpis 1 Poděkování Rád bych poděkoval prof. Ing. Miloši Pejchalovi, CSc. za vedení mé diplomové práce, odborné rady a připomínky, poskytnutí odborné literatury ze své osobní knihovny a za motivaci, která mě vedla k lepšímu výkonu, ochotě a vstřícnosti. Dále bych rád poděkoval svému dědovi Ing. Josefu Schuchovi CSc. za poskytnutí životních zkušeností, pomoc při psaní práce a doprovázení při terénních průzkumech a zároveň děkuji celé mé rodině za podporu. -

Rhododendron Molle (Ericaceae): Phytochemistry, Pharmacology, and Toxicology
Chinese Journal of Natural Chinese Journal of Natural Medicines 2018, 16(6): 04010410 Medicines Rhododendron Molle (Ericaceae): phytochemistry, pharmacology, and toxicology CAI Yong-Qing1Δ, HU Jian-Hui2Δ, QIN Jie3, SUN Tao4*, LI Xiao-Li5,6* 1 Department of Pharmacy, Institute of Surgery Research, Daping Hospital, Third Military Medical University, Chongqing 400042, China; 2 Student Brigade Ten Team, Second Military Medical University, Shanghai 200433, China; 3 Student Eleven Camp, Third Military Medical University, Chongqing 400038, China; 4 College of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China; 5 College of Pharmacy, Chongqing Medical University, Chongqing 400016, China; 6 Chongqing Key Laboratory of Drug Metabolism, Chongqing 400016, China Available online 20 June, 2018 [ABSTRACT] Rhododendron molle G. Don, belonging to the Ericaceae family, is a traditional Chinese medicinal plant with a wide spectrum of pharmacological effects. This paper aimed to review the phytochemistry, pharmacology and toxicology of R. molle, and to discuss the tendency of future investigations on this plant. A systematic review of literature about R. molle was carried out using re- sources including classic books about Chinese herbal medicine, and scientific data bases including CNKI, Pubmed, SciFinder, Scopus, and Web of Science. Over 67 compounds, including diterpenes, triterpenes, flavonoids, and lignans, had been extracted and identified from R. molle. The extracts/monomers isolated from the root, flower and fruits of this plant were used as effective agents for treating pains, inflammatory diseases, hypertension, and pest, etc. In addition, diterpenes, such as rhodojaponin III, were considered as the toxic agents associated with the toxicities of this plant. These findings will be significant for the discovery of new drugs from this plant and full utilization of R. -

An Abstract of the Thesis Of
AN ABSTRACT OF THE THESIS OF Ryan J. Hill for the degree of Master of Science in Horticulture presented on March 2, 2020. Title: Fine Mapping and Gene Expression Analysis of Self-Incompatibility in Hazelnut Abstract approved: ______________________________________________________ Shawn A. Mehlenbacher The European hazelnut (Corylus avellana L.) is a diploid (2n = 2x = 22) tree crop important to the economy of Oregon’s Willamette Valley, where 99% of hazelnut production in the United States is located. Corylus avellana exhibits sporophytic self- incompatibility (SSI), controlled by a single S-locus with at least 33 unique alleles. The alleles exhibit codominance in the stigma and dominance or codominance in the pollen. SSI is present in many plant families and is understood best at the molecular level in Brassica. However, Brassica gene sequences have not proven useful for investigations in Corylus. With new genomic tools available for the study of hazelnut, including a new Pacific Biosciences (PacBio) reference genome for ‘Jefferson’, more progress can be made toward the goal of identifying the genetic determinants of SSI. BAC end sequences associated with the minimal tiling path of the S-locus were used to identify 36 PacBio contigs associated with the hazelnut S-locus (located on linkage group 5[LG5]). Simple sequence repeat (SSR) markers were developed from a subset of dinucleotide repeats found in those contigs and were characterized and mapped. Markers mapping near the S-locus on LG5 were screened against a population of 192 seedlings selected for recombination between the flanking markers of the S-locus, G05-510 and AU02-1350, for which the S-alleles had been determined. -

Comparative Analysis of Microsatellite, SNP, and Indel Markers in Four Rhododendron Species Based on RNA-Seq
Breeding Science 68: 536–544 (2018) doi:10.1270/jsbbs.18092 Research Paper Comparative analysis of microsatellite, SNP, and InDel markers in four Rhododendron species based on RNA-seq Shuzhen Wang, Zhiliang Li, Xudong Guo, Yuanping Fang, Jun Xiang and Weibin Jin* Hubei Key Laboratory of Economic Forest Germplasm Improvement and Resources Comprehensive Utilization; Hubei Collaborative Innovation Center for the Characteristic Resources Exploitation of Dabie Mountains; College of Life Science, Huanggang Normal University, Huanggang, 438000, Hubei Province, P.R. China Rhododendron possesses valuable horticultural and medicinal properties. However, the genetic studies have been hindered due to the lack of genetic markers. Based on RNA-seq, large-scale molecular markers were de- veloped from four Rhododendron species endemic to Dabie Mountains (central China): R. fortunei (5.25 Gb; SSRs, 12,756, one/2.37 kb, 147 types; SNPs, 38,313; InDels, 3,174), R. simsii (5.80 Gb; SSRs, 13,294, one/2.58 kb, 167 types; SNPs, 136,590; InDels, 6,258), R. mariesii (6.53 Gb; SSRs, 15,724, one/2.51 kb, 170 types; SNPs, 44,942; InDels, 4,126), and R. molle (4.35 Gb; SSRs, 10,214, one/2.49 kb, 110 types; SNPs, 77,829; InDels, 3,416). Di-nucleotide repeats were the main type (59.126%–64.314%), and AG/CT repeat (55.18%–61.22%) was the most. In particular, 89 species-specific types had been found. Furthermore, C:G→T:A mutation was the main SNP type (30.475%–34.99%). However, C:G→G:C mutation was the least type in R. -

The Rhododendron Genome and Chromosomal Organization Provide Insight Into Shared Whole-Genome Duplications Across the Heath Family (Ericaceae)
GBE The Rhododendron Genome and Chromosomal Organization Provide Insight into Shared Whole-Genome Duplications across the Heath Family (Ericaceae) Valerie L. Soza 1,*, Dale Lindsley1,†, Adam Waalkes1,5, Elizabeth Ramage1, Rupali P. Patwardhan2, Joshua N. Burton2,6, Andrew Adey2,7,AkashKumar2,8, Ruolan Qiu2,†, Jay Shendure2,3,4, and Benjamin Hall1 1Department of Biology, University of Washington, Seattle, WA 2Department of Genome Sciences, University of Washington, Seattle, WA 3Brotman Baty Institute for Precision Medicine, Seattle, WA 4Howard Hughes Medical Institute, University of Washington, Seattle, WA 5Present address: Department of Laboratory Medicine, University of Washington, Seattle, WA 6Present address: Adaptive Biotechnologies, Seattle, WA 7Present address: Department of Molecular and Medical Genetics, Oregon Health and Science University, Portland, OR 8Present address: Department of Pediatrics, Stanford University, Palo Alto, CA †Retired. *Corresponding author: E-mail: [email protected]. Accepted: November 4, 2019 Data deposition: DNA sequencing data and final genome assembly for Rhododendron williamsianum have been deposited at NCBI BioProject under the accession PRJNA432092 and at CoGe under genome ID 51679. In the genome assembly, within each linkage group, all ordered scaffolds are given in their LACHESIS order, followed by all unordered scaffolds listed in descending order of length. Unclustered scaffolds are listed after linkage group 13 in descending order of length. The scripts used for genome assembly, annotation, and comparative genomics are available in the GitHub repository (https://github.com/vsoza/rhododendron-genome/). Synteny analyses within the Comparative Genomics (CoGe) platform can be obtained from the links given. Rhododendron delavayi: https://genomevolution.org/r/14kzh (whole-genome); Rhododendron williamsia- num: https://genomevolution.org/r/12blb, https://genomevolution.org/r/17qc6 (ordered scaffolds); Vaccinum corymbosum: https://genomevolu- tion.org/r/14oui. -

De Novo Transcriptome Sequencing of Rhododendron Molle and Identification of Genes Involved in the Biosynthesis of Secondary Metabolites Guo-Lin Zhou and Ping Zhu*
Zhou and Zhu BMC Plant Biology (2020) 20:414 https://doi.org/10.1186/s12870-020-02586-y RESEARCH ARTICLE Open Access De novo transcriptome sequencing of Rhododendron molle and identification of genes involved in the biosynthesis of secondary metabolites Guo-Lin Zhou and Ping Zhu* Abstract Background: Rhododendron molle (Ericaceae) is a traditional Chinese medicinal plant, its flower and root have been widely used to treat rheumatism and relieve pain for thousands of years in China. Chemical studies have revealed that R. molle contains abundant secondary metabolites such as terpenoinds, flavonoids and lignans, some of which have exhibited various bioactivities including antioxidant, hypotension and analgesic activity. In spite of immense pharmaceutical importance, the mechanism underlying the biosynthesis of secondary metabolites remains unknown and the genomic information is unavailable. Results: To gain molecular insight into this plant, especially on the information of pharmaceutically important secondary metabolites including grayanane diterpenoids, we conducted deep transcriptome sequencing for R. molle flower and root using the Illumina Hiseq platform. In total, 100,603 unigenes were generated through de novo assembly with mean length of 778 bp, 57.1% of these unigenes were annotated in public databases and 17,906 of those unigenes showed significant match in the KEGG database. Unigenes involved in the biosynthesis of secondary metabolites were annotated, including the TPSs and CYPs that were potentially responsible for the biosynthesis of grayanoids. Moreover, 3376 transcription factors and 10,828 simple sequence repeats (SSRs) were also identified. Additionally, we further performed differential gene expression (DEG) analysis of the flower and root transcriptome libraries and identified numerous genes that were specifically expressed or up-regulated in flower. -
Sorted by Moth Species
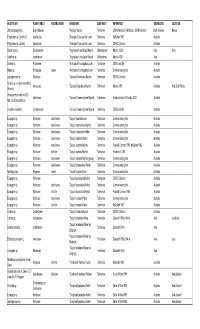
HOST PLANT PLANT FAMILY FEEDING NICHE HERBIVORE SUBFAMILY REFERENCE GEOREGION LOCATION Jatropha gossypifolia Euphorbiaceae "Amorbia" depicta Tortricinae CSIRO Mexican Field Station; USNM collection North America Meixco Polystichum sp. ("prolifera") Aspidiaceae "Anisogona" placoxantha Lower Tortricinae McQuillan 1992 Australia Polystichum sp. (as fern) Aspidiaceae "Anisogona" placoxantha Lower Tortricinae CSIRO Collection Australia Cordia myxa L. Boraginaceae "Argyroploce" cenchropis Meyrick Olethreutinae Meyrick 1920a Asia India Acanthus sp. Acanthaceae "Argyroploce" vinculigera Meyrick Olethreutinae Meyrick 1939 Asia Banksia sp. Proteaceae "Arotrophora" cosmoplaca Lower Tortricinae CSIRO card file Australia Hakea sp. Proteaceae leaves "Arotrophora" cosmoplaca Lower Tortricinae Common rearing files Australia Leptospermum sp. Myrtaceae "Cacoecia" desmotana Meyrick Tortricinae CSIRO Collection Australia Senecio sp. (as plant resembling Asteraceae "Cacoecia" jugicolana Meyrick Tortricinae Meyrick 1881 Australia New South Wales Senecio) Osteospermum ecklonis (DC.) Asteraceae "Cacoecia" mnemosynana Meyrick Tortricinae Herbison-Evans & Crossley 2003 Australia Norl. (as Dimorphotheca) Goodenia ovata Sm. Goodeniaceae "Cacoecia" mnemosynana Meyrick Tortricinae CSIRO card file Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" ceramica Lower Tortricinae Common rearing files Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" cnaphalodes Meyrick Tortricinae Common rearing files Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" constrictana