Sea Lice and Salmon: a Science Primer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Twenty Thousand Parasites Under The
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en Departament de Biologia Animal, Biologia Vegetal i Ecologia Tesis Doctoral Twenty thousand parasites under the sea: a multidisciplinary approach to parasite communities of deep-dwelling fishes from the slopes of the Balearic Sea (NW Mediterranean) Tesis doctoral presentada por Sara Maria Dallarés Villar para optar al título de Doctora en Acuicultura bajo la dirección de la Dra. Maite Carrassón López de Letona, del Dr. Francesc Padrós Bover y de la Dra. Montserrat Solé Rovira. La presente tesis se ha inscrito en el programa de doctorado en Acuicultura, con mención de calidad, de la Universitat Autònoma de Barcelona. Los directores Maite Carrassón Francesc Padrós Montserrat Solé López de Letona Bover Rovira Universitat Autònoma de Universitat Autònoma de Institut de Ciències Barcelona Barcelona del Mar (CSIC) La tutora La doctoranda Maite Carrassón Sara Maria López de Letona Dallarés Villar Universitat Autònoma de Barcelona Bellaterra, diciembre de 2016 ACKNOWLEDGEMENTS Cuando miro atrás, al comienzo de esta tesis, me doy cuenta de cuán enriquecedora e importante ha sido para mí esta etapa, a todos los niveles. -

Catching the Complexity of Salmon-Louse Interactions
Fish and Shellfish Immunology 90 (2019) 199–209 Contents lists available at ScienceDirect Fish and Shellfish Immunology journal homepage: www.elsevier.com/locate/fsi Full length article Catching the complexity of salmon-louse interactions T ∗ Cristian Gallardo-Escáratea, , Valentina Valenzuela-Muñoza, Gustavo Núñez-Acuñaa, Crisleri Carreraa, Ana Teresa Gonçalvesa, Diego Valenzuela-Mirandaa, Bárbara P. Benaventea, Steven Robertsb a Interdisciplinary Center for Aquaculture Research, Laboratory of Biotechnology and Aquatic Genomics, Department of Oceanography, Universidad de Concepción, Concepción, Chile b School of Aquatic and Fishery Sciences (SAFS), University of Washington, Seattle, USA ABSTRACT The study of host-parasite relationships is an integral part of the immunology of aquatic species, where the complexity of both organisms has to be overlayed with the lifecycle stages of the parasite and immunological status of the host. A deep understanding of how the parasite survives in its host and how they display molecular mechanisms to face the immune system can be applied for novel parasite control strategies. This review highlights current knowledge about salmon and sea louse, two key aquatic animals for aquaculture research worldwide. With the aim to catch the complexity of the salmon-louse interactions, molecular information gleaned through genomic studies are presented. The host recognition system and the chemosensory receptors found in sea lice reveal complex molecular components, that in turn, can be disrupted through specific molecules such as non-coding RNAs. 1. Introduction worldwide the most studied sea lice species are Lepeophtheirus salmonis and Caligus rogercresseyi [8–10]. Annually the salmon industry presents Host-parasite interactions are a complex relationship where one estimated losses of US $ 480 million, representing between 4 and 10% organism benefits the other and often where there is a negative impact of production costs [10]. -

Piscirickettsia Salmonis and the Sea Louse Caligus Rogercresseyi
Disease Resistance in Atlantic Salmon (Salmo salar): Coinfection of the Intracellular Bacterial Pathogen Piscirickettsia salmonis and the Sea Louse Caligus rogercresseyi Jean Paul Lhorente1, Jose´ A. Gallardo2*, Beatriz Villanueva3, Marı´a J. Caraban˜ o3, Roberto Neira1,4 1 Aquainnovo S.A, Puerto Montt, Chile, 2 Pontificia Universidad Cato´lica de Valparaı´so, Valparaı´so, Chile, 3 Departamento de Mejora Gene´tica Animal, INIA, Madrid, Spain, 4 Departamento de Produccio´n Animal, Facultad de Ciencias Agrono´micas, Universidad de Chile, Santiago, Chile Abstract Background: Naturally occurring coinfections of pathogens have been reported in salmonids, but their consequences on disease resistance are unclear. We hypothesized that 1) coinfection of Caligus rogercresseyi reduces the resistance of Atlantic salmon to Piscirickettsia salmonis; and 2) coinfection resistance is a heritable trait that does not correlate with resistance to a single infection. Methodology: In total, 1,634 pedigreed Atlantic salmon were exposed to a single infection (SI) of P. salmonis (primary pathogen) or coinfection with C. rogercresseyi (secondary pathogen). Low and high level of coinfection were evaluated (LC = 44 copepodites per fish; HC = 88 copepodites per fish). Survival and quantitative genetic analyses were performed to determine the resistance to the single infection and coinfections. Main Findings: C. rogercresseyi significantly increased the mortality in fish infected with P. salmonis (SI mortality = 251/545; LC mortality = 544/544 and HC mortality = 545/545). Heritability estimates for resistance to P. salmonis were similar and of 2 2 2 medium magnitude in all treatments (h SI = 0.2360.07; h LC = 0.1760.08; h HC = 0.2460.07). A large and significant genetic correlation with regard to resistance was observed between coinfection treatments (rg LC-HC = 0.9960.01) but not between the single and coinfection treatments (rg SI-LC = 20.1460.33; rg SI-HC = 0.3260.34). -

Host-Parasite Interaction of Atlantic Salmon (Salmo Salar) and the Ectoparasite Neoparamoeba Perurans in Amoebic Gill Disease
ORIGINAL RESEARCH published: 31 May 2021 doi: 10.3389/fimmu.2021.672700 Host-Parasite Interaction of Atlantic salmon (Salmo salar) and the Ectoparasite Neoparamoeba perurans in Amoebic Gill Disease † Natasha A. Botwright 1*, Amin R. Mohamed 1 , Joel Slinger 2, Paula C. Lima 1 and James W. Wynne 3 1 Livestock and Aquaculture, CSIRO Agriculture and Food, St Lucia, QLD, Australia, 2 Livestock and Aquaculture, CSIRO Agriculture and Food, Woorim, QLD, Australia, 3 Livestock and Aquaculture, CSIRO Agriculture and Food, Hobart, TAS, Australia Marine farmed Atlantic salmon (Salmo salar) are susceptible to recurrent amoebic gill disease Edited by: (AGD) caused by the ectoparasite Neoparamoeba perurans over the growout production Samuel A. M. Martin, University of Aberdeen, cycle. The parasite elicits a highly localized response within the gill epithelium resulting in United Kingdom multifocal mucoid patches at the site of parasite attachment. This host-parasite response Reviewed by: drives a complex immune reaction, which remains poorly understood. To generate a model Diego Robledo, for host-parasite interaction during pathogenesis of AGD in Atlantic salmon the local (gill) and University of Edinburgh, United Kingdom systemic transcriptomic response in the host, and the parasite during AGD pathogenesis was Maria K. Dahle, explored. A dual RNA-seq approach together with differential gene expression and system- Norwegian Veterinary Institute (NVI), Norway wide statistical analyses of gene and transcription factor networks was employed. A multi- *Correspondence: tissue transcriptomic data set was generated from the gill (including both lesioned and non- Natasha A. Botwright lesioned tissue), head kidney and spleen tissues naïve and AGD-affected Atlantic salmon [email protected] sourced from an in vivo AGD challenge trial. -
Lake Huron Spawning
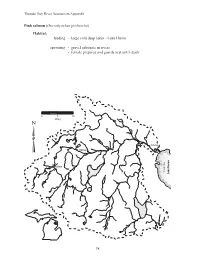
Thunder Bay River Assessment Appendix Pink salmon (Oncorhynchus gorbuscha) Habitat: feeding - large cold deep lakes - Lake Huron spawning - gravel substrate in rivers - female prepares and guards nest until death 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 98 Thunder Bay River Assessment Appendix Coho salmon (Oncorhynchus kisutch) Habitat: feeding - adults: Lake Huron - young: shallow gravel substrate in cold streams, later into pools spawning - cold streams and rivers - swifter water of shallow gravelly substrate 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 99 Thunder Bay River Assessment Appendix Rainbow trout (Oncorhynchus mykiss) Habitat: feeding - cold clear water of rivers and Lake Huron - moderate current spawning - gravelly riffles above a pool - smaller tributaries 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 100 Thunder Bay River Assessment Appendix Chinook salmon (Oncorhynchus tshawyscha) Habitat: feeding - adults: Lake Huron - young: shallow gravel substrate in cool streams, later into pools spawning - gravelly substrate in cool streams 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 101 Thunder Bay River Assessment Appendix Round whitefish (Prosopium cylindraceum) Habitat: feeding - lakes, rivers, and streams spawning - shallows of lakes and rivers - gravel or rock substrate 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 102 Thunder Bay River Assessment Appendix Atlantic salmon (Salmo salar) Habitat: feeding - young: gravel substrate streams - adults: Lake Huron -

Salmon Fact Sheet
THE WILD SALMON SEAFOOD MARKET’S GUIDE TO W I L D P A C I F I C S A L M O N Salmon Pacific Salmon occur from northern California along the Pacific Coast throughout the Pacific Ocean, Bering Sea and Arctic Ocean waters adjacent to Alaska. Salmon are anadromous, that is, they spawn in fresh water and the young migrate to the sea where they mature. The mature Salmon returns to the stream of their birth to spawn. Nutrition Few single foods bring as many valuable contributions to the table as Salmon. An excellent source of high-quality protein, containing all the essential amino acids. The fats in Salmon are predominately unsaturated. These fats are evidenced to reduce the risk of heart disease. Availability Although each species has a particular season, small fisheries of wild salmon occur periodically, making fresh salmon (often hard to find and expensive) available throughout the year. Your best values will come during peak salmon season, May through September. Frozen salmon (often frozen at sea) is available during the off season. Also known as Chinook Salmon. Also known as Silver Salmon. Highly desired for King The largest of the species and the most Coho both table use and smoking. Coho salmon offers prominent of the salmon known for its high oil firm meat with excellent flavor slightly milder than content and distinctive, rich flavor. King and Sockeye. Average size from 5 to 40 lbs. Average size from 4 - 9 lbs. Available May - September Available June - September Copper River & Yukon River King Also known as Chum Salmon. -

Imagine the Silver Beauty and the Fighting Spirit of Atlantic Salmon; The
Sakhalin Silver Text and Photos: Clemens Ratschan Imagine the silver beauty and the fighting spirit of Atlantic salmon; the complex, unpredictable life- history of sea trout and combine with the ferocious take and body mass of a predatory taimen. This will give you a glimpse of what fishing for Sakhalin taimen, the silver of the Russian Far East, is about. AM PLEASED TO introduce Siberian taimen, Hucho taimen. No this fish to the readers of wonder, scientists also erroneously Chasing Silver, because in related this far-eastern species to many respects it forms a the large-sized, non-anadromous missing link between the predators of the genus Hucho, which Ifishery for anadromous salmon and is a branch of the salmonoid tree for huchen, a big predatory non- that occurs exclusively in Eurasia. anadromous salmonoid in my home In Central Europe, Hucho hucho is country of Austria (‘Danube salmon’ restricted to the Danube System, in English. See article “Taimen” by where self-sustaining stocks are Wolfgang Hauer, issue 3/2010). presently only found in a handful of Sakhalin taimen is one of the rivers in Germany, Austria, Slovakia least-known salmonid species among and former Yugoslavia. Huchen is non-Russian fishermen; even many very closely related to the already- Russians tend to confuse it with the mentioned Siberian taimen. The latter | 62 | Chasing Silver Fly Fishing Magazine April’s Fav Five www.chasingsilvermagazine.com | 63 | Sakhalin Silver inhabits a distant, vast range from a habits. But one ecological feature expeditions to Japan. Later, the fish few places in European Russia to the is unique – all members of the true was assigned to the genus Parahucho, Lena and Amur rivers in the very far huchen live exclusively in fresh water, with regard to some obvious east of northern Asia. -

Inventory of Parasitic Copepods and Their Hosts in the Western Wadden Sea in 1968 and 2010
INVENTORY OF PARASITIC COPEPODS AND THEIR HOSTS IN THE WESTERN WADDEN SEA IN 1968 AND 2010 Wouter Koch NNIOZIOZ KKoninklijkoninklijk NNederlandsederlands IInstituutnstituut vvooroor ZZeeonderzoekeeonderzoek INVENTORY OF PARASITIC COPEPODS AND THEIR HOSTS IN THE WESTERN WADDEN SEA IN 1968 AND 2010 Wouter Koch Texel, April 2012 NIOZ Koninklijk Nederlands Instituut voor Zeeonderzoek Cover illustration The parasitic copepod Lernaeenicus sprattae (Sowerby, 1806) on its fish host, the sprat (Sprattus sprattus) Copyright by Hans Hillewaert, licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license; CC-BY-SA-3.0; Wikipedia Contents 1. Summary 6 2. Introduction 7 3. Methods 7 4. Results 8 5. Discussion 9 6. Acknowledgements 10 7. References 10 8. Appendices 12 1. Summary Ectoparasites, attaching mainly to the fins or gills, are a particularly conspicuous part of the parasite fauna of marine fishes. In particular the dominant copepods, have received much interest due to their effects on host populations. However, still little is known on the copepod fauna on fishes for many localities and their temporal stability as long-term observations are largely absent. The aim of this project was two-fold: 1) to deliver a current inventory of ectoparasitic copepods in fishes in the southern Wadden Sea around Texel and 2) to compare the current parasitic copepod fauna with the one from 1968 in the same area, using data published in an internal NIOZ report and additional unpublished original notes. In total, 47 parasite species have been recorded on 52 fish species in the southern Wadden Sea to date. The two copepod species, where quantitative comparisons between 1968 and 2010 were possible for their host, the European flounder (Platichthys flesus), showed different trends: Whereas Acanthochondria cornuta seems not to have altered its infection rate or per host abundance between years, Lepeophtheirus pectoralis has shifted towards infection of smaller hosts, as well as to a stronger increase of per-host abundance with increasing host length. -

Full Text in Pdf Format
Vol. 27: 277–287, 2015 ENDANGERED SPECIES RESEARCH Published online May 13 doi: 10.3354/esr00675 Endang Species Res OPENPEN ACCESSCCESS Causes of the drastic loss of genetic variation in the Critically Endangered Formosa landlocked salmon of Taiwan Te-Hua Hsu1, Keisuke Takata2, Hiroshi Onozato3, Jin-Chywan Gwo1,* 1Department of Aquaculture, National Taiwan Ocean University, Keelung 20224, Taiwan 2Faculty of Science, Shinshu University, Matsumoto-city, Nagano 390-8621, Japan 3Matsumoto Institute of Microorganisms Co. Ltd., Matsumoto-city, Nagano 390-1241, Japan ABSTRACT: The use of hatchery-reared fish to replenish existing threatened wild populations has been shown to reduce or change the natural genetic diversity of the wild populations. In this study, the genetic diversity of wild Formosa landlocked salmon Oncorhynchus formosanus in its main habitat of the Chichiawan Stream in Taiwan was examined after a large-scale escape of hatchery- cultivated fish. Approximately 3000 individuals (the descendants of only 5 pairs of wild salmon) es- caped from an old hatchery when Typhoon Ariel breached the hatchery in the fall of 2004. The ge- netic diversity of the wild population was extremely low at that time, and declined further between 2004 and 2008 following the escape of hatchery fish. We hypothesize that the decline in genetic di- versity of the wild population was mainly caused by a population bottleneck in 2005, and that ge- netic homogeneity since 2005 was caused by breeding of the escaped hatchery fish (which showed low genetic diversity) that survived the floods of 2004. This supports the possibility that the drastic decline in genetic diversity between 2004 and 2008 was caused by the genetic effects of the escaped hatchery fish, and demonstrates the risk of introducing hatchery fish into the wild. -

Morphological and Phylogenetic Analysis of Caligus Spp Isolated from Lates Calcarifer Cultured in Floating Net Cages in Malaysia
MORPHOLOGICAL AND PHYLOGENETIC ANALYSIS OF CALIGUS SPP ISOLATED FROM LATES CALCARIFER CULTURED IN FLOATING NET CAGES IN MALAYSIA MUHD FAIZUL HELMI BIN AHAMAD HASMI FACULTY OF SCIENCE UNIVERSITY OF MALAYA KUALA LUMPUR 2013 MORPHOLOGICAL AND PHYLOGENETIC ANALYSIS OF CALIGUS SPP ISOLATED FROM LATES CALCARIFER CULTURED IN FLOATING NET CAGES IN MALAYSIA MUHD FAIZUL HELMI BIN AHAMAD HASMI DISSERTATION SUBMITTED IN FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE INSTITUTE OF BIOLOGICAL SCIENCES FACULTY OF SCIENCE UNIVERSITY OF MALAYA KUALA LUMPUR 2013 UNIVERSITI MALAYA ORIGINAL LITERARY WORK DECLARATION Name of Candidate: MUHD FAIZUL HELMI BIN AHAMAD HASMI I/C/Passport No: 850809045109 Regisration/Matric No.: SGR090128 Name of Degree: MASTER OF SCIENCE Title of Project Paper/Research Report/Dissertation/Thesis (“this Work”): “MORPHOLOGICAL AND PHYLOGENETIC ANALYSIS OF CALIGUS SPP ISOLATED FROM LATES CAILCARIFER CULTURED IN FLOATING NET CAGES IN MALAYSIA” Field of Study: MOLECULAR PARASITOLOGY I do solemnly and sincerely declare that: (1) I am the sole author/writer of this Work, (2) This Work is original, (3) Any use of any work in which copyright exists was done by way of fair dealing and for permitted purposes and any excerpt or extract from, or reference to or reproduction of any copyright work has been disclosed expressly and sufficiently and the title of the Work and its authorship have been acknowledged in this Work, (4) I do not have any actual knowledge nor do I ought reasonably to know that the making of this work -

APPENDIX 1 Classified List of Fishes Mentioned in the Text, with Scientific and Common Names
APPENDIX 1 Classified list of fishes mentioned in the text, with scientific and common names. ___________________________________________________________ Scientific names and classification are from Nelson (1994). Families are listed in the same order as in Nelson (1994), with species names following in alphabetical order. The common names of British fishes mostly follow Wheeler (1978). Common names of foreign fishes are taken from Froese & Pauly (2002). Species in square brackets are referred to in the text but are not found in British waters. Fishes restricted to fresh water are shown in bold type. Fishes ranging from fresh water through brackish water to the sea are underlined; this category includes diadromous fishes that regularly migrate between marine and freshwater environments, spawning either in the sea (catadromous fishes) or in fresh water (anadromous fishes). Not indicated are marine or freshwater fishes that occasionally venture into brackish water. Superclass Agnatha (jawless fishes) Class Myxini (hagfishes)1 Order Myxiniformes Family Myxinidae Myxine glutinosa, hagfish Class Cephalaspidomorphi (lampreys)1 Order Petromyzontiformes Family Petromyzontidae [Ichthyomyzon bdellium, Ohio lamprey] Lampetra fluviatilis, lampern, river lamprey Lampetra planeri, brook lamprey [Lampetra tridentata, Pacific lamprey] Lethenteron camtschaticum, Arctic lamprey] [Lethenteron zanandreai, Po brook lamprey] Petromyzon marinus, lamprey Superclass Gnathostomata (fishes with jaws) Grade Chondrichthiomorphi Class Chondrichthyes (cartilaginous -

Observing Copepods Through a Genomic Lens James E Bron1*, Dagmar Frisch2, Erica Goetze3, Stewart C Johnson4, Carol Eunmi Lee5 and Grace a Wyngaard6
Bron et al. Frontiers in Zoology 2011, 8:22 http://www.frontiersinzoology.com/content/8/1/22 DEBATE Open Access Observing copepods through a genomic lens James E Bron1*, Dagmar Frisch2, Erica Goetze3, Stewart C Johnson4, Carol Eunmi Lee5 and Grace A Wyngaard6 Abstract Background: Copepods outnumber every other multicellular animal group. They are critical components of the world’s freshwater and marine ecosystems, sensitive indicators of local and global climate change, key ecosystem service providers, parasites and predators of economically important aquatic animals and potential vectors of waterborne disease. Copepods sustain the world fisheries that nourish and support human populations. Although genomic tools have transformed many areas of biological and biomedical research, their power to elucidate aspects of the biology, behavior and ecology of copepods has only recently begun to be exploited. Discussion: The extraordinary biological and ecological diversity of the subclass Copepoda provides both unique advantages for addressing key problems in aquatic systems and formidable challenges for developing a focused genomics strategy. This article provides an overview of genomic studies of copepods and discusses strategies for using genomics tools to address key questions at levels extending from individuals to ecosystems. Genomics can, for instance, help to decipher patterns of genome evolution such as those that occur during transitions from free living to symbiotic and parasitic lifestyles and can assist in the identification of genetic mechanisms and accompanying physiological changes associated with adaptation to new or physiologically challenging environments. The adaptive significance of the diversity in genome size and unique mechanisms of genome reorganization during development could similarly be explored.