CHEMICAL KINETICS Fybsc I) DEFINITIONS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Chemistry –II
Subject Chemistry Paper No and Title Paper 5: Organic Chemistry –II Module No and Title Module 5: Methods of determining mechanisms and Isotope effects Module Tag CHE_P5_M5_e-Text CHEMISTRY PAPER No. 5: Organic Chemistry -II MODULE No. 5: Methods of determining mechanisms and Isotope effects TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Methods of determining mechanism 3.1 Determination of the products formed 3.2 Study of Intermediate formed 3.3 Study of catalyst 3.4 Stereochemical Evidence 3.5 Kinetic evidence 3.6 Isotope Labelling 4. Isotopic Effects 5. Summary CHEMISTRY PAPER No. 5: Organic Chemistry -II MODULE No. 5: Methods of determining mechanisms and Isotope effects 1. Learning Outcomes After studying this module, you shall be able to • Know what do we mean by mechanism of a reaction • Learn the ways to determine mechanism of a reaction • Identify the reactants, products and intermediates involved in a reaction • Evaluate the steps involved in a reaction 2. Introduction In scientific experiments and chemical reactions, all we can do is try to account for the observations by proposing theories and mechanisms. Reaction mechanisms have been an integral part of the teaching of organic chemistry and in the planning of routes for organic syntheses for about 50 years. The first sentence of Hammett’s influential book, Physical Organic Chemistry, states, “A major part of the job of the chemist is the prediction and control of the course of chemical reactions” In a chemical reaction, mechanism depicts the actual process by which the reaction has taken place. It indicates which bonds are broken, in what order, the steps involved and the relative rate of each step. -

Chapter 12 Lecture Notes
Chemical Kinetics “The study of speeds of reactions and the nanoscale pathways or rearrangements by which atoms and molecules are transformed to products” Chapter 13: Chemical Kinetics: Rates of Reactions © 2008 Brooks/Cole 1 © 2008 Brooks/Cole 2 Reaction Rate Reaction Rate Combustion of Fe(s) powder: © 2008 Brooks/Cole 3 © 2008 Brooks/Cole 4 Reaction Rate Reaction Rate + Change in [reactant] or [product] per unit time. Average rate of the Cv reaction can be calculated: Time, t [Cv+] Average rate + Cresol violet (Cv ; a dye) decomposes in NaOH(aq): (s) (mol / L) (mol L-1 s-1) 0.0 5.000 x 10-5 13.2 x 10-7 10.0 3.680 x 10-5 9.70 x 10-7 20.0 2.710 x 10-5 7.20 x 10-7 30.0 1.990 x 10-5 5.30 x 10-7 40.0 1.460 x 10-5 3.82 x 10-7 + - 50.0 1.078 x 10-5 Cv (aq) + OH (aq) → CvOH(aq) 2.85 x 10-7 60.0 0.793 x 10-5 -7 + + -5 1.82 x 10 change in concentration of Cv Δ [Cv ] 80.0 0.429 x 10 rate = = 0.99 x 10-7 elapsed time Δt 100.0 0.232 x 10-5 © 2008 Brooks/Cole 5 © 2008 Brooks/Cole 6 1 Reaction Rates and Stoichiometry Reaction Rates and Stoichiometry Cv+(aq) + OH-(aq) → CvOH(aq) For any general reaction: a A + b B c C + d D Stoichiometry: The overall rate of reaction is: Loss of 1 Cv+ → Gain of 1 CvOH Rate of Cv+ loss = Rate of CvOH gain 1 Δ[A] 1 Δ[B] 1 Δ[C] 1 Δ[D] Rate = − = − = + = + Another example: a Δt b Δt c Δt d Δt 2 N2O5(g) 4 NO2(g) + O2(g) Reactants decrease with time. -

Ochem ACS Review 23 Aryl Halides
ACS Review Aryl Halides 1. Which one of the following has the weakest carbon-chlorine bond? A. I B. II C. III D. IV 2. Which compound in each of the following pairs is the most reactive to the conditions indicated? A. I and III B. I and IV C. II and III D. II and IV 3. Which of the following reacts at the fastest rate with potassium methoxide (KOCH 3) in methanol? A. fluorobenzene B. 4-nitrofluorobenzene C. 2,4-dinitrofluorobenzene D. 2,4,6-trinitrofluorobenzene 4. Which of the following reacts at the fastest rate with potassium methoxide (KOCH 3) in methanol? A. fluorobenzene B. p-nitrofluorobenzene C. p-fluorotoluene D. p-bromofluorobenzene 5. Which of the following is the kinetic rate equation for the addition-elimination mechanism of nucleophilic aromatic substitution? A. rate = k[aryl halide] B. rate = k[nucleophile] C. rate = k[aryl halide][nucleophile] D. rate = k[aryl halide][nucleophile] 2 6. Which of the following is not a resonance form of the intermediate in the nucleophilic addition of hydroxide ion to para -fluoronitrobenzene? A. I B. II C. III D. IV 7. Which chlorine is most susceptible to nucleophilic substitution with NaOCH 3 in methanol? A. #1 B. #2 C. #1 and #2 are equally susceptible D. no substitution is possible 8. What is the product of the following reaction? A. N,N-dimethylaniline B. para -chloro-N,N-dimethylaniline C. phenyllithium (C 6H5Li) D. meta -chloro-N,N-dimethylaniline 9. Which one of the reagents readily reacts with bromobenzene without heating? A. -

Enzymatic Kinetic Isotope Effects from First-Principles Path Sampling
Article pubs.acs.org/JCTC Enzymatic Kinetic Isotope Effects from First-Principles Path Sampling Calculations Matthew J. Varga and Steven D. Schwartz* Department of Chemistry and Biochemistry, University of Arizona, Tucson, Arizona 85721, United States *S Supporting Information ABSTRACT: In this study, we develop and test a method to determine the rate of particle transfer and kinetic isotope effects in enzymatic reactions, specifically yeast alcohol dehydrogenase (YADH), from first-principles. Transition path sampling (TPS) and normal mode centroid dynamics (CMD) are used to simulate these enzymatic reactions without knowledge of their reaction coordinates and with the inclusion of quantum effects, such as zero-point energy and tunneling, on the transferring particle. Though previous studies have used TPS to calculate reaction rate constants in various model and real systems, it has not been applied to a system as large as YADH. The calculated primary H/D kinetic isotope effect agrees with previously reported experimental results, within experimental error. The kinetic isotope effects calculated with this method correspond to the kinetic isotope effect of the transfer event itself. The results reported here show that the kinetic isotope effects calculated from first-principles, purely for barrier passage, can be used to predict experimental kinetic isotope effects in enzymatic systems. ■ INTRODUCTION staging method from the Chandler group.10 Another method that uses an energy gap formalism like QCP is a hybrid Particle transfer plays a crucial role in a breadth of enzymatic ff reactions, including hydride, hydrogen atom, electron, and quantum-classical approach of Hammes-Schi er and co-work- 1 ers, which models the transferring particle nucleus as a proton-coupled electron transfer reactions. -

Materials Science
Materials Science Materials Science is an interdisciplinary field combining physics (fundamental laws of nature), chemistry (interactions of atoms) and biology (how life interacts with materials) to elucidate the inherent properties of basic and complex systems. This includes optical (interaction with light), electrical (interaction with charge), magnetic and structural properties of everyday electronics, clothing and architecture. The Materials Science central dogma follows the sequence: Structure—Properties—Design—Performance. This involves relating the nanostructure of a material to its macroscale physical and chemical properties. By understanding and then changing the structure, material scientists can create custom materials with unique properties. The goal of the materials science minor is to create a cross-disciplinary approach to fundamental topics in basic and applied physical sciences. Students will gain experience and perspectives from the disciplines of chemistry, physics and biology. The minor places a strong emphasis on current nanoscale research methods in addition to the basics of electronic, optical and mechanical properties of materials. Any student with an interest in pursuing the cross-disciplinary minor in materials science should consult with the coordinator of the minor. Students are encouraged to declare their participation in their sophomore year but no later than the end of the junior year. Students also should seek an adviser from participating faculty. Degree Requirements for the Minor General College requirements -

S.T.E.T.Women's College, Mannargudi Semester Iii Ii M
S.T.E.T.WOMEN’S COLLEGE, MANNARGUDI SEMESTER III II M.Sc., CHEMISTRY ORGANIC CHEMISTRY - II – P16CH31 UNIT I Aliphatic nucleophilic substitution – mechanisms – SN1, SN2, SNi – ion-pair in SN1 mechanisms – neighbouring group participation, non-classical carbocations – substitutions at allylic and vinylic carbons. Reactivity – effect of structure, nucleophile, leaving group and stereochemical factors – correlation of structure with reactivity – solvent effects – rearrangements involving carbocations – Wagner-Meerwein and dienone-phenol rearrangements. Aromatic nucleophilic substitutions – SN1, SNAr, Benzyne mechanism – reactivity orientation – Ullmann, Sandmeyer and Chichibabin reaction – rearrangements involving nucleophilic substitution – Stevens – Sommelet Hauser and von-Richter rearrangements. NUCLEOPHILIC SUBSTITUTION Mechanism of Aliphatic Nucleophilic Substitution. Aliphatic nucleophilic substitution clearly involves the donation of a lone pair from the nucleophile to the tetrahedral, electrophilic carbon bonded to a halogen. For that reason, it attracts to nucleophile In organic chemistry and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which a leaving group(nucleophile) is replaced by an electron rich compound(nucleophile). The whole molecular entity of which the electrophile and the leaving group are part is usually called the substrate. The nucleophile essentially attempts to replace the leaving group as the primary substituent in the reaction itself, as a part of another molecule. The most general form of the reaction may be given as the following: Nuc: + R-LG → R-Nuc + LG: The electron pair (:) from the nucleophile(Nuc) attacks the substrate (R-LG) forming a new 1 bond, while the leaving group (LG) departs with an electron pair. The principal product in this case is R-Nuc. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. -

2. Electrochemistry
EP&M. Chemistry. Physical chemistry. Electrochemistry. 2. Electrochemistry. A phenomenon of electric current in solid conductors (class I conductors) is a flow of electrons caused by the electric field applied. For this reason, such conductors are also known as electronic conductors (metals and some forms of conducting non-metals, e.g., graphite). The same phenomenon in liquid conductors (class II conductors) is a flow of ions caused by the electric field applied. For this reason, such conductors are also known as ionic conductors (solutions of dissociated species and molten salts, known also as electrolytes). The question arises, what is the phenomenon causing the current flow across a boundary between the two types of conductors (known as an interface), i.e., when the circuit looks as in Fig.1. DC source Fig. 1. A schematic diagram of an electric circuit consis- flow of electrons ting of both electronic and ionic conductors. The phenomenon occuring at the interface must involve both the electrons and ions to ensure the continuity of the circuit. metal metal flow of ions ions containing solution When a conventional redox reaction occurs in a solution, the charge transfer (electron transfer) happens when the two reacting species get in touch with each other. For example: Ce4+ + Fe 2+→ Ce 3+ + Fe 3+ (2.1) In this reaction cerium (IV) draws an electron from iron (II) that leads to formation of cerium (III) and iron (III) ions. Cerium (IV), having a strong affinity for electrons, and therefore tending to extract them from other species, is called an oxidizing agent or an oxidant. -

Physical Chemistry MEHAU KULYK / SCIENCE PHOTO LIBRARY LIBRARY PHOTO SCIENCE / KULYK MEHAU
Physical chemistry MEHAU KULYK / SCIENCE PHOTO LIBRARY LIBRARY PHOTO SCIENCE / KULYK MEHAU 44 | Chemistry World | August 2010 www.chemistryworld.org Let’s get physical Physical chemists are finding themselves more in demand than ever. Emma Davies finds out why Physical chemistry is entering theory in harmony, he said: ‘with the called Fueling the future, his team something of a golden era. Its overall perspective of contributing In short is using IR spectroscopy to see tools have advanced dramatically accurate, experimentally vetted, The field of physical how protons are accommodated in recent years, so much so that molecular level pictures of reactive chemistry is booming, as in imidazole nanostructures. The scientists from all disciplines are pathways and relevant structures, more and more scientists project is based at the University of entering collaborations with physical physical chemists are in an excellent seek to understand their Massachussetts at Amhurst, US and chemists to gain new insight into position to engage chemistry in work on a molecular level teams are currently working on fuel their specialist subject areas. There is all of its complexity.’ He believes Developing alternative cells containing alternatives to nafion, however some worry that the subject that understanding processes at a energy sources is one a fluoropolymer-copolymer which is could become a victim of its own molecular level is crucial to making area benefiting from a good proton conductor but fails in success, with fundamental research grand scientific leaps forward. a physical chemistry meeting contemporary demand for losing out in the funding stakes to Johnson and his team are working approach high temperature operation. -

Syllabus Chem 646
SYLLABUS CHEM 646 Course title and number Physical Organic Chemistry, CHEM 646 Term Fall 2019 Meeting times and location MWF 10:20 am – 11:10 am, Room: CHEM 2121 Course Description and Learning Outcomes Prerequisites: Organic Chemistry I and II or equivalent undergraduate organic chemistry courses. Physical Organic Chemistry (CHEM646) is a graduate/senior-undergrad level course of advanced organic chemistry. Physical organic chemistry refers to a discipline of organic chemistry that focuses on the relationship between chemical structure and property/reactivity, in particular, applying experimental and theoretical tools of physical chemistry to the study of organic molecules and reactions. Specific focal points of study include the bonding and molecular orbital theory of organic molecules, stability of organic species, transition states, and reaction intermediates, rates of organic reactions, and non-covalent aspects of solvation and intermolecular interactions. CHEM646 is designed to prepare students for graduate research on broadly defined organic chemistry. This course will provide the students with theoretical and practical frameworks to understand how organic structures impact the properties of organic molecules and the mechanism for organic reactions. By the end of this course, students should be able to: 1. Gain in-depth understanding of the nature of covalent bonds and non-covalent interactions 2. Use molecule orbital theory to interpret the property and reactivity of organic species. 3. Understand the correlation between the structure and physical/chemical properties of organic molecules, such as stability, acidity, and solubility. 4. Predict the reactivity of organic molecules using thermodynamic and kinetic analyses 5. Probe the mechanism of organic reactions using theoretical and experimental approaches. -

Alkyl Halides
Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp3 orbital of an alkyl group. CHCl3 (Chloroform: organic solvent) CF2Cl2 (Freon-12: refrigerant CFC) CF3CHClBr (Halothane: anesthetic) Halogen atoms are more electronegative than carbon atoms, and so the C-Hal bond is polarized. The C-Hal (often written C-X) bond is polarized in such a way that there is partial positive charge on the carbon and partial negative charge on the halogen. Ch06 Alkyl Halides (landscape).docx Page 1 Dipole moment Electronegativities decrease in the order of: F > Cl > Br > I Carbon-halogen bond lengths increase in the order of: C-F < C-Cl < C-Br < C-I Bond Dipole Moments decrease in the order of: C-Cl > C-F > C-Br > C-I = 1.56D 1.51D 1.48D 1.29D Typically the chemistry of alkyl halides is dominated by this effect, and usually results in the C-X bond being broken (either in a substitution or elimination process). This reactivity makes alkyl halides useful chemical reagents. Ch06 Alkyl Halides (landscape).docx Page 2 Nomenclature According to IUPAC, alkyl halides are treated as alkanes with a halogen (Halo-) substituent. The halogen prefixes are Fluoro-, Chloro-, Bromo- and Iodo-. Examples: Often compounds of CH2X2 type are called methylene halides. (CH2Cl2 is methylene chloride). CHX3 type compounds are called haloforms. (CHI3 is iodoform). CX4 type compounds are called carbon tetrahalides. (CF4 is carbon tetrafluoride). Alkyl halides can be primary (1°), secondary (2°) or tertiary (3°). Other types: A geminal (gem) dihalide has two halogens on the same carbon. -

CHEMICAL KINETICS Pt 2 Reaction Mechanisms Reaction Mechanism
Reaction Mechanism (continued) CHEMICAL The reaction KINETICS 2 C H O +→5O + 6CO 4H O Pt 2 3 4 3 2 2 2 • has many steps in the reaction mechanism. Objectives ! Be able to describe the collision and Reaction Mechanisms transition-state theories • Even though a balanced chemical equation ! Be able to use the Arrhenius theory to may give the ultimate result of a reaction, determine the activation energy for a reaction and to predict rate constants what actually happens in the reaction may take place in several steps. ! Be able to relate the molecularity of the reaction and the reaction rate and • This “pathway” the reaction takes is referred to describle the concept of the “rate- as the reaction mechanism. determining” step • The individual steps in the larger overall reaction are referred to as elementary ! Be able to describe the role of a catalyst and homogeneous, heterogeneous and reactions. enzyme catalysis Reaction Mechanisms Often Used Terms •Intermediate: formed in one step and used up in a subsequent step and so is never seen as a product. The series of steps by which a chemical reaction occurs. •Molecularity: the number of species that must collide to produce the reaction indicated by that A chemical equation does not tell us how step. reactants become products - it is a summary of the overall process. •Elementary Step: A reaction whose rate law can be written from its molecularity. •uni, bi and termolecular 1 Elementary Reactions Elementary Reactions • Consider the reaction of nitrogen dioxide with • Each step is a singular molecular event carbon monoxide. -
Microtest III Print File
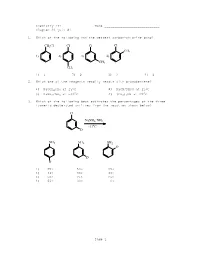
Chemistry 211 Name ____________________________ Chapter 23 Quiz #1 1. Which of the following has the weakest carbon-chlorine bond? CH2Cl Cl Cl Cl CH3 1) 2) 3) 4) CH3 CH3 1) 1 2) 2 3) 3 4) 4 2. Which one of the reagents readily reacts with bromobenzene? 1) NaOCH2CH3 at 250C 2) NaCN/DMSO at 250C 3) NaNH2/NH3 at -330C 4) (CH3)2NH at 250C 3. Which of the following best estimates the percentages of the three isomeric deuterated anilines from the reaction shown below? Cl NaNH2, NH3 -33oC D NH 2 NH 2 NH 2 D D D 1) 25% 50% 25% 2) 33% 33% 33% 3) 50% 25% 25% 4) 66% 33% 0% Page 1 4. Which of the following is (are) true concerning the intermediate in the addition-elimination mechanism of the reaction below? F OCH3 NaOCH 3 intermediate NO2 NO2 A. The intermediate is aromatic. B. The intermediate is a resonance stabilized anion. C. Electron withdrawing groups on the benzene ring stabilize the intermediate. 1) only A 2) only B 3) A and C 4) B and C 5. Carbon-14 labelled chlorobenzene is reacted with sodium amide in ammonia as shown below. Which of the following depicts the carbon-14 label in the product(s)? Cl NaNH2/NH3 -33oC Cl Cl Cl 1) 3) and (about 50% of each) Cl Cl Cl 2) 4) and (about 50% of each) 1) 1 2) 2 3) 3 4) 4 6. Which of the following reacts at the fastest rate with potassium methoxide (KOCH3) in methanol? 1) fluorobenzene 2) 4-nitrofluorobenzene 3) 2,4-dinitrofluorobenzene 4) 2,4,6-trinitrofluorobenzene Page 2 7.