Peak Expiratory Flow at Altitude Thorax: First Published As 10.1136/Thx.45.8.620 on 1 August 1990
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Standardisation of Spirometry
Eur Respir J 2005; 26: 319–338 DOI: 10.1183/09031936.05.00034805 CopyrightßERS Journals Ltd 2005 SERIES ‘‘ATS/ERS TASK FORCE: STANDARDISATION OF LUNG FUNCTION TESTING’’ Edited by V. Brusasco, R. Crapo and G. Viegi Number 2 in this Series Standardisation of spirometry M.R. Miller, J. Hankinson, V. Brusasco, F. Burgos, R. Casaburi, A. Coates, R. Crapo, P. Enright, C.P.M. van der Grinten, P. Gustafsson, R. Jensen, D.C. Johnson, N. MacIntyre, R. McKay, D. Navajas, O.F. Pedersen, R. Pellegrino, G. Viegi and J. Wanger CONTENTS AFFILIATIONS Background ............................................................... 320 For affiliations, please see Acknowledgements section FEV1 and FVC manoeuvre .................................................... 321 Definitions . 321 CORRESPONDENCE Equipment . 321 V. Brusasco Requirements . 321 Internal Medicine University of Genoa Display . 321 V.le Benedetto XV, 6 Validation . 322 I-16132 Genova Quality control . 322 Italy Quality control for volume-measuring devices . 322 Fax: 39 103537690 E-mail: [email protected] Quality control for flow-measuring devices . 323 Test procedure . 323 Received: Within-manoeuvre evaluation . 324 March 23 2005 Start of test criteria. 324 Accepted after revision: April 05 2005 End of test criteria . 324 Additional criteria . 324 Summary of acceptable blow criteria . 325 Between-manoeuvre evaluation . 325 Manoeuvre repeatability . 325 Maximum number of manoeuvres . 326 Test result selection . 326 Other derived indices . 326 FEVt .................................................................. 326 Standardisation of FEV1 for expired volume, FEV1/FVC and FEV1/VC.................... 326 FEF25–75% .............................................................. 326 PEF.................................................................. 326 Maximal expiratory flow–volume loops . 326 Definitions. 326 Equipment . 327 Test procedure . 327 Within- and between-manoeuvre evaluation . 327 Flow–volume loop examples. 327 Reversibility testing . 327 Method . -

Spirometry (Adult) Guideline
Document Number # QH-GDL-386:2012 Spirometry (Adult) Respiratory Science Custodian/Review Officer: 1. Purpose Chief Allied Health Officer This guideline provides recommendations regarding best practice to support high quality spirometry practice Version no: 1.0 throughout Queensland Health facilities. Applicable To: 2. Scope All Health Practitioners performing adult This guideline provides information for all health spirometry practitioners who perform adult spirometry as part of their clinical duties. Approval Date: 26/11/2012 This guideline provides the minimum requirements for obtaining acceptable and repeatable pre- and post- Effective Date: 26/11/2012 bronchodilator (reversibility) spirometric data using both volume and flow-measuring devices. Next Review Date: 26/11/2013 3. Related documents Authority: This guideline is primarily based on the following Chair – State-wide Clinical Measurements Network documents taken from the series “ATS/ERS Task Force: Standardisation of Lung Function Testing” Approving Officer General considerations for lung function testing 1 Chief Allied Health Officer (2005). Standardisation of spirometry (2005). 2 References from alternate sources of information have Supersedes: Nil been identified in this document. Key Words: spirometry, spiro, respiratory, Policy and Standard/s: measure, spirogram, spirometric, bronchodilator, flow-volume loop, peak Informed Decision-making in Healthcare (QH-POL- flow 346:2011) 3 Accreditation References: Procedures, Guidelines, Protocols EQuIP and other criteria and standards Australian Guidelines for the prevention and control of infection in healthcare (CD33:2010) 4 2005 American Thoracic Society and European Respiratory Society (ATS/ERS) guidelines 1, 2 Version No.: 1.0; Effective From: 26 November 2012 Page 1 of 31 Printed copies are uncontrolled Queensland Health: Spriometry (Adult) Queensland Health Paediatric Spirometry Guideline Forms and templates Nil 4. -

Pulmonary Function Test (PFT)
Pulmonary Function Test (PFT) St. James Mercy Hospital Respiratory Therapy, 1st Floor 411 Canisteo St. Hornell, NY 14843 607-324- 8159 To schedule an appointment: 607-324-2879 To fax to scheduling: 607-324-8221 What is pulmonary function testing and why is it done? Pulmonary function tests (also called PFTs or lung function tests) help determine how well your lungs are functioning. The results of these tests tell your physician how much air your lungs can hold, how quickly you can move air into and out of your lungs, and how well your lungs are able to use oxygen and get rid of carbon dioxide. The tests help your physician determine if you have a lung disease, help provide a measure of how significant your lung disease is, and can show how well the treatment for your lung disease is working. How is a pulmonary function test done? Pulmonary function testing is usually done by a specially trained respiratory therapist or technician. For most pulmonary function tests, you will be asked to wear a nose clip to make sure that no air passes through your nose during the test. You will be asked to breathe into a mouthpiece that is connected to a machine called a spirometer. The technician may encourage you to breathe deeply during parts of the test to get the best results. Following all of the technician's instructions will help provide the most accurate results. How do I prepare for my test? You should not eat a heavy meal just before this test. You should not smoke for six hours before the test. -

Effect of Chronic Bronchitis on Changes in Pulmonary Function Caused by Irradiation Ofthe Lungs
Thorax: first published as 10.1136/thx.20.4.303 on 1 July 1965. Downloaded from Thorax (1965), 20, 303. Effect of chronic bronchitis on changes in pulmonary function caused by irradiation of the lungs B. I. HOFFBRAND,1 P. M. S. GILLAM,' AND P. J. D. HEAF' From the Chest Department, University College Hospital, London In a previous paper (Gillam, Heaf, Hoffbrand, and Group 3: A history of chronic cough with Hilton, 1964) it was shown that, contrary to wide- sputum and recurrent chest infections or breath- spread opinion, the presence of co-existing chronic lessness or both. This group will be referred to as bronchitis does not interfere with the treatment of the severe bronchitic group. A subgroup 3a was bronchial carcinoma by radiotherapy. Patients derived from group 3 and consisted of those with severe chronic bronchitis can be given the patients in group 3 with a forced expiratory same dose of irradiation as those with mild volume in one second (F.E.V.j.0) of 1,250 ml. or bronchitis or with previously normal lungs. less. Group 3a thus consisted largely of those Survival after radiotherapy for bronchial carci- patients with the severest bronchitis of all. noma is not shortened by co-existing chronic Group 1 was similar to groups 2 and 3 in bronchitis. The incidence of exacerbations of respect of age, sex, previous lung resections, and bronchitis both during and after radiotherapy is radiotherapy received. Group 1, however, con- no more than might be expected in such a group tained a higher proportion of patients with more extensive and more anaplastic tumours. -

Breathing and Lung Function Tests Tests to Measure Your Breathing
Breathing and lung function tests Tests to measure your breathing What are breathing tests? Breathing tests measure how well your lungs are working. These tests are used to find the cause of problems such as feeling out of breath. They are sometimes called lung function tests. They measure things like: • how much air you can take into your lungs • how quickly you can blow air out of your lungs • how well your lungs can take up oxygen • the strength of your breathing muscles Your results are compared to what would be expected in healthy people of your age, height, sex and ethnicity. Your health care professional may also weigh you to calculate your body mass index (BMI) (nhs.uk/live-well/healthy-weight/bmi-calculator/ ). This is a way of measuring whether you’re a healthy weight for your height. Being overweight or underweight can affect your breathing, lung function and general health. Being overweight can cause breathlessness. Your health care professional will also compare your results with any earlier test results, to track your progress. Tests include: • peak flow test • spirometry and bronchodilator responsiveness (reversibility) test • lung volume measurement • gas transfer test • respiratory muscle strength • exhaled carbon monoxide levels • fractional exhaled nitric oxide testing (FeNO) Peak flow test What is a peak flow test? The peak flow test measures how fast you can breathe out after you’ve taken a full breath in. Your peak flow score is sometimes called your peak expiratory flow (PEF). Breathing and lung function tests 1 blf.org.uk/breathing-tests Your GP or nurse should ask you to do a peak flow test at your annual asthma review. -

Respiratory Health Spirometry Procedures Manual
Respiratory Health Spirometry Procedures Manual January 2008 TABLE OF CONTENTS Chapter Page 1 SPIROMETRY OVERVIEW.......................................................................... 1-1 1.1 Overview of the Spirometry Exam Component.................................. 1-1 1.2 The Bronchodilator Subcomponent .................................................... 1-2 1.3 Chronic Obstructive Pulmonary Disease............................................ 1-3 1.4 Asthma................................................................................................ 1-4 1.5 Basics of Respiration .......................................................................... 1-5 1.6 Spirometric Measurements ................................................................. 1-6 ACKNOWLEDGMENTS AND REFERENCES............................................ 1-13 2 EQUIPMENT AND SUPPLIES...................................................................... 2-1 2.1 List of Equipment and Supplies.......................................................... 2-1 2.2 Description of Equipment and Supplies ............................................. 2-2 2.2.1 Equipment ........................................................................... 2-2 2.2.2 Supplies............................................................................... 2-3 2.3 Equipment Setup Procedures .............................................................. 2-4 2.3.1 Equipment ........................................................................... 2-4 2.3.2 Supplies.............................................................................. -

SPIROMETRY a Spirometer Is a Vital Tool in the Measurement of Lung Functions. It Provides Important Information to Help Diagnose
SPIROMETRY A spirometer is a vital tool in the measurement of lung functions. It provides important information to help diagnose, manage and treat a patient with any lung disease including asthma, and COPD (chronic obstructive pulmonary disease: emphysema, and chronic bronchitis). Most children by five years of age can perform a spirometry. The cost of owning a spirometer has been decreasing and insurance companies, including Medicare and Medicaid will reimburse the physician for performing the spirometry and the interpretation, so in a short time the spirometer will pay for itself. In the future we will see that a pulmonary function testing will be required to be done for all patients with a diagnosis of cough, bronchitis, wheezing, emphysema, pneumonia or any respiratory disorder. Diagnosing asthma early at its onset is very important in helping reduce the patient’s lung function over time. Asthma is a chronic disease and early treatment can keep a person breathing normal for life, unfortunately poorly controlled asthma can lead to COPD, which is chronic emphysema and chronic bronchitis. Monitoring someone with asthma or COPD with a spirometer can inform a physician on when to increase or even decrease medication. There are great videos on the internet, including you tube describing how to perform a spirometry, as well as interpreting a spirometry. Reading and interpreting the spirometry data is simple. The most important pieces of information are the FVC, FEV1, FEV1/FVC ratio, and FEF 25-75 percentages. The data is given in actual measurement of volumes in liters, but one should just focus on the percentages. -

Effects of Position, Dead Air Space and Exercise on Breathing
Effects of Position, Dead Air Space and Exercise on Breathing Background information Spirometry is a diagnostic technique used to measure respiratory volumes. The instrument used to measure these volumes is called spirometer. The four pulmonary volumes include tidal volume, inspiratory reserve volume, expiratory reserve volume, and residual volume. The lung volumes for a patient are usually compared with predicted lung volumes based on age, sex, and body size. There are also four standard lung capacities, which consist of two or more standard lung volume in combination. The four lung capacities are inspiratory capacity, vital capacity, functional residual capacity, and total lung capacity. The volume of air that enters or leaves the lungs during one breathing cycle is called the tidal volume (TV). Normally, the amount of air that is expired during a breathing cycle is equal to the amount of air that is inspired during the cycle. Resting tidal volume is the amount exchanged during normal, resting breathing (eupnea). According to Levitzky (2007), “during normal, quiet breathing (eupnea) the tidal volume of a 70-kg adult is about 500 mL per breath, but this volume can increase dramatically, for example, during exercise”. During times of increased demand, for example, during exercise, the tidal volume can be increased using some of the inspiratory or expiratory reserve lung volume to bring more fresh air into the body. The volume of gas that is inhaled into the lungs during a maximal forced inspiration starting at the end of a normal tidal inspiration is called the inspiratory reserve volume (IRV). It is determined by the strength of contraction of the inspiratory muscles, inward elastic recoil of the lung and the chest wall. -
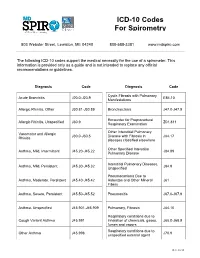
ICD-10 Codes for Spirometry
ICD-10 Codes For Spirometry 803 Webster Street, Lewiston, ME 04240 800-588-3381 www.mdspiro.com The following ICD-10 codes support the medical necessity for the use of a spirometer. This information is provided only as a guide and is not intended to replace any official recommendations or guidelines. Diagnosis Code Diagnosis Code Cystic Fibrosis with Pulmonary Acute Bronchitis J20.0-J20.9 E84.10 Manifestations Allergic Rhinitis, Other J30.81-J30.89 Bronchiectasis J47.0-J47.9 Encounter for Preprocedural Allergic Rhinitis, Unspecified J30.9 Z01.811 Respiratory Examination Other Interstitial Pulmonary Vasomotor and Allergic J30.0-J30.5 Disease with Fibrosis in J84.17 Rhinitis diseases classified elsewhere Other Specified Interstitial Asthma, Mild, Intermittent J45.20-J45.22 J84.89 Pulmonary Disease Interstitial Pulmonary Diseases, Asthma, Mild, Persistent J45.30-J45.32 J84.9 Unspecified Pneumoconiosis Due to Asthma, Moderate, Persistent J45.40-J45.42 Asbestos and Other Mineral J61 Fibers Asthma, Severe, Persistent J45.50-J45.52 Pneumonitis J67.0-J67.9 Asthma, Unspecified J45.901-J45.909 Pulmonary, Fibrosis J84.10 Respiratory conditions due to Cough Variant Asthma J45.991 inhalation of chemicals, gases, J68.0-J68.9 fumes and vapors Respiratory conditions due to Other Asthma J45.998 J70.9 unspecified external agent Rev. 10/15 Diagnosis Code Diagnosis Code Other Long Term (Current) Sarcoidosis of the Lung D86.0 Z79.899 Drug Therapy Sarcoidosis of the Lung with D86.2 Shortness of Breath R06.02 sacoidosis of the lymph nodes Systemic Sclerosis -

Forced Spirometry and Related Tests
© Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION OneCHAPTER 1 Forced Spirometry and Related Tests ᭤ Introduction 1 ᭤ Physiology 2 ᭤ Spirometry Instrumentation 7 ᭤ Spirometry Testing Techniques 24 ᭤ Calculations and Reporting Results 36 ᭤ Reversibility Testing 50 ᭤ Basic Elements of Interpretation 52 ᭤ Special Considerations 55 ᭤ Maximum Voluntary Ventilation 56 ᭤ Peak Expiratory Flow Rate Monitoring 57 ᭤ Infection Control 58 ᭤ Case Presentations 59 ᭤ Self-Assessment Questions 66 Introduction Forced spirometry, often referred to as spirometry, is an essential component in the medical evaluation of patients complaining of shortness of breath. It is also widely used to evaluate the benefi cial effects and adverse reactions of therapeutic interventions and to monitor the effects of environmental and occupational exposures. In addition, forced spirometry is used in disability/ impairment evaluations, in preoperative assessments, and to monitor lung function over time. Spirometry has shown considerable growth in the past 30 years, for several reasons: (a) published standards and testing guidelines,1-6 (b) improved spirometers and software, (c) evi- dence that both patients and physicians have inaccurate perceptions of the severity of airfl ow obstruction,7-11 (d ) evidence that history taking and physical examination by themselves are not 1 c01.indd 1 5/26/2011 6:27:10 PM © Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION 2 CHAPTER 1 Forced Spirometry and Related Tests helpful in identifying patterns of lung disease,12,13 (e) recommendations that spirometry be included in the assessment of patients suspected of having asthma,14,15 and recommendations for objective measurements to reduce the impact of chronic obstructive pulmonary disease (COPD).16 This chapter will provide information for both the student and the practitioner by discuss- ing relevant physiology, instrumentation, techniques of performance and calculations, and basic elements of interpretation. -

Spirometry Performance and Interpretation for Healthcare Professionals 2015
SPIROMETRY Performance and Interpretation for Healthcare Professionals Faculty of Respiratory Physiology IICMS Version 1 – April 2015 Authors: Maria Mc Neill & Geraldine Nolan www.iars.ie Contents Page Background 3 What is Spirometry? 3 Definitions 3 Who should get a Spirometry test? 4 Who performs Spirometry? 4 Quality Spirometry 5 Equipment performance criteria 5 Quality Control including calibration & verification 6 Measurement procedure 7 Summary of within and between acceptability criteria for test 10 Spirometry graphs and tracings 11 Reversibility testing 13 Interpretation 14 Differential diagnosis of COPD vs Asthma 15 Guide to interpreting the results 16 Diagnostic flow chart 18 References 19 2 Background Faculty of Respiratory Physiology of Irish Institute of Clinical Measurement Science, with the endorsement of the Irish Thoracic Society (ITS), perform spirometry to the ATS/ERS task force (2005 update): Standardisation of Spirometry, guidelines1. This document is based on these guidelines and will supersede the previous ITS document on Spirometry performance and interpretation2. What is Spirometry? Spirometry is a physiological test that measures how an individual inhales or exhales volumes of air as a function of time. The primary signal measured in spirometry may be volume or flow. Spirometry is invaluable as a screening test of general respiratory health in the same way that blood pressure provides important information about general cardiovascular health. However, on its own, spirometry does not lead clinicians directly to an aetiological diagnosis. Spirometry can be undertaken with many different types of equipment, and requires cooperation between the subject and the examiner, and the results obtained will depend on technical as well as personal factors. -

Respiratory Health Spirometry Procedures Manual
Respiratory Health Spirometry Procedures Manual January 2011 TABLE OF CONTENTS Chapter Page 1 SPIROMETRY OVERVIEW .......................................................................... 1-1 1.1 Overview of the Spirometry Exam Component .................................. 1-1 1.2 The Bronchodilator Subcomponent .................................................... 1-2 1.3 Chronic Obstructive Pulmonary Disease ............................................ 1-3 1.4 Asthma ................................................................................................ 1-4 1.5 Basics of Respiration .......................................................................... 1-5 1.6 Spirometric Measurements ................................................................. 1-6 ACKNOWLEDGMENTS AND REFERENCES ............................................ 1-13 2 EQUIPMENT AND SUPPLIES ...................................................................... 2-1 2.1 List of Equipment and Supplies .......................................................... 2-1 2.2 Description of Equipment and Supplies ............................................. 2-2 2.2.1 Equipment ........................................................................... 2-2 2.2.2 Supplies ............................................................................... 2-3 2.3 Equipment Setup Procedures .............................................................. 2-4 2.3.1 Equipment ........................................................................... 2-4 2.3.2 Supplies ..............................................................................