Download Article (Pdf)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Functional School in 2011-12 Madhya Pradesh S No. District
List of Functional School in 2011-12 Madhya Pradesh S No. District Block 1 Ashok Nagar Mungaoli 2 Ashok Nagar Eshagarh 3 Bhopal Berasia 4 Burhanpur Khaknar 5 Burhanpur Burhanpur 6 Chhatarpur Gaurihar 7 Chhatarpur Bijawar 8 Chhatarpur Buxwaha 9 Chhindwara Tamia 10 Chhindwara Amarwada 11 Chhindwara Parasia 12 Chhindwara Jamai 13 Gwalior Bhitarwar 14 Gwalior Ghatigaon 15 Harda Khirkiya 16 Hoshangabad Sohagpur 17 Hoshangabad Babai 18 Hoshangabad Bankhedi 19 Jabalpur Shahpura 20 Khandwa Harsud 21 Khandwa Pamdhana 22 Khandwa Baladi (Killod) 23 Panna Panna 24 Panna Pawai 25 Sagar Banda 26 Sagar Bina 27 Sagar Khurai 28 Sagar Malthon 29 Sagar Shahgarh 30 Vidisha Basoda 31 Vidisha Nataran 32 Vidisha Sirong 33 Vidisha Latari Alirajpur ( Jhabua Dist in 34 Alirajpur MDM List ) Bhabra(Jhabua Dist in MDM 35 Alirajpur List ) Jobat(Jhabua Dist in MDM 36 Alirajpur List ) Katthiwada(Jhabua Dist in 37 Alirajpur MDM List ) Sondwa (Jhabua Dist in MDM 38 Alirajpur List ) Udaigarh(Jhabua Dist in MDM 39 Alirajpur List ) Anuppur (SHAHDOL is in 40 Anuppur MDM list ) Jaithahri((SHAHDOL is in 41 Anuppur MDM list ) Kotma(SHAHDOL is in MDM 42 Anuppur list ) Pushaprajgarh(SHAHDOL is 43 Anuppur in MDM list ) 44 Balaghat Baihar 45 Balaghat Birsa 46 Barwani Barwani 47 Barwani Niwali 48 Barwani Pansemal 49 Barwani Pati 50 Barwani Rajpur 51 Barwani Sendhwa 52 Betul Bhimpur 53 Betul Ghoradongari 54 Betul Shahpur 55 Chhindwara Harrai 56 Damoh Tendukheda 57 Dewas Kannod 58 Dewas Khategaon 59 Dhar Badnawar 60 Dhar Bagh 61 Dhar Dahi 62 Dhar Dhar 63 Dhar Dharampuri 64 Dhar Gandhwani -
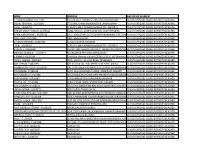
Name Address Nature of Payment P
NAME ADDRESS NATURE OF PAYMENT P. NAVEENKUMAR -91774443 NO 139 KALATHUMEDU STREETMELMANAVOOR0 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED VISHAL TEKRIWAL -31262196 27,GOPAL CHANDRAMUKHERJEE LANEHOWRAH CLAIMS CHEQUES ISSUED BUT NOT ENCASHED LOCAL -16280591 #196 5TH MAIN ROADCHAMRAJPETPH 26679019 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED BHIKAM SINGH THAKUR -21445522 JABALPURS/O UDADET SINGHVILL MODH PIPARIYA CLAIMS CHEQUES ISSUED BUT NOT ENCASHED ATINAINARLINGAM S -91828130 NO 2 HINDUSTAN LIVER COLONYTHAGARAJAN STREET PAMMAL0CLAIMS CHEQUES ISSUED BUT NOT ENCASHED USHA DEVI -27227284 VPO - SILOKHARA00 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED SUSHMA BHENGRA -19404716 A-3/221,SECTOR-23ROHINI CLAIMS CHEQUES ISSUED BUT NOT ENCASHED LOCAL -16280591 #196 5TH MAIN ROADCHAMRAJPETPH 26679019 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED RAKESH V -91920908 NO 304 2ND FLOOR,THIRUMALA HOMES 3RD CROSS NGRLAYOUT,CLAIMS CHEQUES ROOPENA ISSUED AGRAHARA, BUT NOT ENCASHED KRISHAN AGARWAL -21454923 R/O RAJAPUR TEH MAUCHITRAKOOT0 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED K KUMAR -91623280 2 nd floor.olympic colonyPLOT NO.10,FLAT NO.28annanagarCLAIMS west, CHEQUES ISSUED BUT NOT ENCASHED MOHD. ARMAN -19381845 1571, GALI NO.-39,JOOR BAGH,TRI NAGAR0 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED ANIL VERMA -21442459 S/O MUNNA LAL JIVILL&POST-KOTHRITEH-ASHTA CLAIMS CHEQUES ISSUED BUT NOT ENCASHED RAMBHAVAN YADAV -21458700 S/O SURAJ DEEN YADAVR/O VILG GANDHI GANJKARUI CHITRAKOOTCLAIMS CHEQUES ISSUED BUT NOT ENCASHED MD SHADAB -27188338 H.NO-10/242 DAKSHIN PURIDR. AMBEDKAR NAGAR0 CLAIMS CHEQUES ISSUED BUT NOT ENCASHED MD FAROOQUE -31277841 3/H/20 RAJA DINENDRA STREETWARD NO-28,K.M.CNARKELDANGACLAIMS CHEQUES ISSUED BUT NOT ENCASHED RAJIV KUMAR -13595687 CONSUMER APPEALCONSUMERCONSUMER CLAIMS CHEQUES ISSUED BUT NOT ENCASHED MUNNA LAL -27161686 H NO 524036 YARDS, SECTOR 3BALLABGARH CLAIMS CHEQUES ISSUED BUT NOT ENCASHED SUNIL KUMAR -27220272 S/o GIRRAJ SINGHH.NO-881, RAJIV COLONYBALLABGARH CLAIMS CHEQUES ISSUED BUT NOT ENCASHED DIKSHA ARORA -19260773 605CELLENO TOWERDLF IV CLAIMS CHEQUES ISSUED BUT NOT ENCASHED R. -

271120191113351031248261119
Data under the Central Sector Scheme of Scholarship for College and University Students S.No.. Application ID Board Name Applicant Name Father's Name Categroy Gender Annual Applicant's Applicant's Applicant's Institute Name Institute Address Applicant's Applicant's Renewal Application Status Family Address District Domicile State Mobile Email Status Income 1 MP201819008150636 BOARD OF SECONDARY SAPNA PANCHAL MAHESH OBC F 35000 WARD NO 5 AGARMALWA MADHYA MATA JEEJA BAI moti tabela indore (m.p.) 9424074178 sapnapanchal62 Renewed Application Under EDUCATION , BHOPAL PANCHAL MACHALPURA PRADESH GOVT. P.G. GIRLS [email protected] Process for ROAD DIST COLLEGE MOTI Verification at AGARMALWA TABELA, INDORE Institute Level MP 2 MP201819000786710 BOARD OF SECONDARY RAJU PRAJAPATI BAISAKHU SC M 40000 JANUNA ANUPPUR MADHYA GOVT. Civil Lines - Pachpedi, 9179792038 praju6181@gm Renewed Application Under EDUCATION , BHOPAL PRAJAPATI COLLIERY WARD PRADESH AUTONMOUS Jabalpur ail.com Process for NO 05 ANUPPUR SCIENCE COLLEGE, Verification at CIVIL LINESR, Institute Level 3 MP201819008030317 BOARD OF SECONDARY SOURABH MAHESH SINGH GENERAL M 40000 GRAM DERKA ASHOKNAGAR MADHYA GOVT. NEHRU Govt. Nehru Degree 7828270629 SOURABHRAGH Renewed Application Under EDUCATION , BHOPAL RAGHUWANSHI ASHOKNAGAR PRADESH DEGREE COLLEGE College, Near Bypass, UWANSHI@GM Process for Eshagarh Road, AIL.COM Verification at Ashoknagar, Ashoknagar Institute Level 4 MP201819008715789 BOARD OF SECONDARY PRIYAM JAIN pawan jain GENERAL F 50000 ward no 4 jain ASHOKNAGAR MADHYA GOVT. NEHRU Govt. Nehru Degree 8827902908 Renewed Application Under EDUCATION , BHOPAL mandir ke pass PRADESH DEGREE COLLEGE College, Near Bypass, Process for piprai Eshagarh Road, Verification at Ashoknagar, Ashoknagar Institute Level 5 MP201819001892162 BOARD OF SECONDARY RISHI KUMAR SAHU shambhu dayal OBC M 40000 near jain ASHOKNAGAR MADHYA NATIONAL National Institute of 8878134349 iitianrishi50@g Renewed Application Under EDUCATION , BHOPAL sahu tempaln ,gram PRADESH INSTITUTE OF Technology Raipur G.E. -
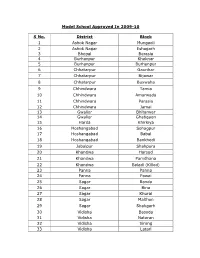
Model School Approved in 2009-10 S No. District Block 1 Ashok Nagar
Model School Approved In 2009-10 S No. District Block 1 Ashok Nagar Mungaoli 2 Ashok Nagar Eshagarh 3 Bhopal Berasia 4 Burhanpur Khaknar 5 Burhanpur Burhanpur 6 Chhatarpur Gaurihar 7 Chhatarpur Bijawar 8 Chhatarpur Buxwaha 9 Chhindwara Tamia 10 Chhindwara Amarwada 11 Chhindwara Parasia 12 Chhindwara Jamai 13 Gwalior Bhitarwar 14 Gwalior Ghatigaon 15 Harda Khirkiya 16 Hoshangabad Sohagpur 17 Hoshangabad Babai 18 Hoshangabad Bankhedi 19 Jabalpur Shahpura 20 Khandwa Harsud 21 Khandwa Pamdhana 22 Khandwa Baladi (Killod) 23 Panna Panna 24 Panna Pawai 25 Sagar Banda 26 Sagar Bina 27 Sagar Khurai 28 Sagar Malthon 29 Sagar Shahgarh 30 Vidisha Basoda 31 Vidisha Nataran 32 Vidisha Sirong 33 Vidisha Latari Model School Approved In 2011-12 S. No. District Block 1. Alirajpur Alirajpur ( Jhabua Dist in MDM List ) 2. Alirajpur Bhabra(Jhabua Dist in MDM List ) 3. Alirajpur Jobat(Jhabua Dist in MDM List ) 4. Alirajpur Katthiwada(Jhabua Dist in MDM List ) 5. Alirajpur Sondwa (Jhabua Dist in MDM List ) 6. Alirajpur Udaigarh(Jhabua Dist in MDM List ) 7. Anuppur Anuppur (SHAHDOL is in MDM list ) 8. Anuppur Jaithahri((SHAHDOL is in MDM list ) 9. Anuppur Kotma(SHAHDOL is in MDM list ) 10. Anuppur Pushaprajgarh(SHAHDOL is in MDM list ) 11. Ashoknagar Ashoknagar (SHAHDOL is in MDM list ) 12. Ashoknagar Chanderi (SHAHDOL is in MDM list ) 13. Balaghat Baihar 14. Balaghat Birsa 15. Barwani Barwani 16. Barwani Niwali 17. Barwani Pansemal 18. Barwani Pati 19. Barwani Rajpur 20. Barwani Sendhwa 21. Betul Bhimpur 22. Betul Ghoradongari 23. Betul Shahpur 24. Bhind Gohad 25. Bhopal Phanda (VIDISHA is in MDM List ) 26. -

SQM Programme Oct2018.Pdf
TECH QC T-6 S-59 izfr] egkizca/kda ¼leLr½ Jh----------------------------------------------------------- e-iz- xzkeh.k lM+d fodkl izkf/kdj.k LVsV DokfyVh ekWfuVj ¼leLr½ ifj;kstuk fØ;kUo;u bdkbZ] ---------------------------------------------------------------- ---------------------------------------------------------------- &&&&&&&&&&&&&&&&&¼e-iz-½ fo"k;%& LVsV DokfyVh ekfuVlZ dk fujh{k.k dk;ZØe ekg vDVwcj&2018A && && 00 iz/kkuea=h xzke lM+d ;kstuk ds ,oa vU; dk;kZs ds Second Tier Quality Monitoring fujh{k.k gsrq ekg vDVwcj&2018 dk fujh{k.k dk;ZØe layXu gS %& 1- fujh{k.k gsrq ftyk vUrxZr fu;qDr fd;s x;s SQM dh lwph layXu gSA 2- fujh{k.k gsrq MPRCP ds ekxksZ dh lwph Geo Reach Software ij ,oa PMGSY vUrxZr ekxksZa dh lwph] ,l-D;w-,e- ds eksckbZy gsaM lsV ij izkIr gksxhA lHkh ,l-D;w-,e- dks MPRCP ds ekxksZ dk fujh{k.k Geo Reach Software ds ek/;e ls ,oa PMGSY vUrxZr ekxksZa dk fujh{k.k eksckbZy Qksu ds ek/;e ls gh fd;k tkuk vfuok;Z gSA PMGSY ekxksZ ds laca/k esa ;g lqfuf’pr gks fd muds eksckbZy Qksu ij ekxZ dh lwph viyksM dh tk pqdh gSA fujh{k.k mijkar fd;s x;s fujh{k.k dh fjiksVZ OMMAS lkbZV ij vfuok;Z :i ls Upload djsaA 3- ,u-D;w-,e- ,oa ,l-D;w-,e- dh yafcr ,-Vh-vkj- dk fujkdj.k izkFkfedrk ij fd;k tkosA 4- OMMAS lkbZV ij ,l-D;w-lh- }kjk viyksM fd, x, ekxksZ dks izkFkfedrk ds vk/kkj ij fujh{k.k djk;k tkosA mDr ekxksZ dk fujh{k.k u fd;s tkus ij bls xaHkhjrk ls fy;k tkosxk&d`i;k uksV djssA lkbV ij viyksM fd;s x;s ekxksZ dh izkFkfedrk Geo reach bdkbZ ds egkizca/kd }kjk fu/kkZfjr dh tkosxhA bl dk;kZy; ds i= Ø- 13151 fnukad 23- 05-17 }kjk dsoy o -

District Census Handbook, Gwalior, Part XIII-B, Series-11
~TIT XIII-~ ~"tTGJ;rT ~T • sn~fq~ ~T\ttr • iti. m. Ii, "1\(f1q srmuf;{1ti ~"t fifbrcti at,,~, ~rot 1)81, 'ERSUS-PUBLJCATION PLAN (1981 Cen.rus Publications, Series 11 in All India Series will be published in the following parIS) GOVERNMENT OF INDIA PUBLICATIONS Part I-A Administration Report- Enumeration Part I-B Administration Report-Tabulation Part II-A General Population Tables P:ut U-B Pr imary Census Abstract Part III General Economic Tables Part IV Social and Cultural Tables Part V Migration Tables Part VI Fertility Tables Part VII Tables on Houses and Disabled Population P:lIt VIII Household Tables Part IX Special Tables on Scheduled Caltes and Schedlued Tribes Part X-A T own Directory Part X-B Survey Reports on selected Towns Part X-C Survey Reports on selected Villages Part XI Ethnographic Notes and special studies ()n Scheduled Castes and Scheduled Tribes, Part XU . CensUS Atlas Paper I of 198,2 Primary Census Abstract for Scheduled Castes and Scheduled Tribes Pa per I of 198-' Household Population by Religion of Head of Hous.ebold. STA fE GOVERNMENT PUBLICATIONS Part XIlI-A&B District Cellsus Handbook for each of the 45 districts in the State. (Village and Town Directory and Primary Cen,susAbUralOt) fq\1t( ,:q) CONTENTS ~ISO Pages Fbreword j-iv Preface v-vi Map of Gwalior District Important Statistics vii Analytical Note 1Slfr~'11f~<Ii' fcCCfI1;t, ~~~f:qcr ;jfrTcr ~!~ 9;[~~f'T.fq Notes & E.<planations: LIst of Scheduled ~'f~rfij 'fiT ~'CfT (~'!lT)a<f), fqa-l1Cfi 1 979; Castes and Scheduled Tribes Order f:srt:JJ ~'f~QFfT ~ffffCfit -

Rdwr New.Xlsx
ादेशक मौसम पूवानुमान के Regional Weather Forecasting Centre ादेशक मौसम के Regional Meteorological Centre भारत मौसम वान वभाग India Meteorological Department नागपुर Nagpur Regional Daily Weather Report Sunday, 18 July 2021 Issue Time : 12:00 Hrs. IST Monsoon Watch ♦ The western part of the Monsoon Trough at mean sea level now passes through Ganganagar, Narnaul, Aligarh, Kanpur, Daltonganj, Shantiniketan, Cherrapunji and thence eastwards to Nagaland and extends upto 0.9 km above mean sea level.♦ A north-south trough runs at 0.9 km above mean sea level from central Madhya Pradesh to south Tamilnadu across Vidarbha, Telangana and Rayalaseema. Main Weather Observations Heavy to very heavy rainfall observed at isolated places over West madhya Pradesh. Very light to moderate rainfall observed at isolated places over Madhya Pradesh and Chhattisgarh. Very light to light rainfall observed at isolated places over Vidarbha. Thunderstorm observed at isolated places over Madhya Pradesh and Chhattisgarh. Rainfall(cm) "Chief Amount of Rainfall (1cm & above) : West Madhya Pradesh: Narwar (dist Shivpuri) 14, Bhitarwar (dist Gwalior) 9, Bairad (dist Shivpuri) 6, Sheopur- (dist Sheopur) 6, Gormi (dist Bhind) 5, Dabra (dist Gwalior) 4, Karera (dist Shivpuri) 4, Gwalior (dist Gwalior) 4, Alipur(jaura) (dist Morena) 3, Karhal (dist Sheopur) 3, Pohri (dist Shivpuri) 3,Shivpuri (dist Shivpuri) 3, Kolaras (dist Shivpuri) 3, Vijaypur (adp) (dist Sheopur) 2, Isagarh (distAshoknagar) 2, Pichhore (dist Shivpuri) 2, Ghatigaon (dist Gwalior) 2, Badoda (dist Sheopur) 2,Badarwas (dist Shivpuri) 1, Morena- (dist Morena) 1, Veerpur (dist Sheopur) 1, Sabalgarh (dist Morena) 1, Datia- (dist Datia) 1, Seodha(seondha) (dist Datia) 1 East Madhya Pradesh : Badwara (dist Katni) 3, Karanjia (dist Dindori) 3, Pushprajgarh (dist Anuppur) 2, Amarpatan (dist Satna) 1, Maihar (dist Satna) 1, Bajag (dist Dindori) 1 Vidarbha: Khamgaon (dist Buldhana) 1, Chhattisgarh:- Jaijaipur (dist Janjgir) 2, Abhanpur (dist Raipur) 2, Kansabel (dist Jashpur) 1, Marwahi (dist Bilaspur) 1, Kusmi (dist Balrampur) 1. -

E-Tender-Cum-Auction for Sale of Lease of Mig Residential Quarter and Land at Darpan Colony, Gwalior, Mp
E-TENDER-CUM-AUCTION FOR SALE OF LEASE OF MIG RESIDENTIAL QUARTER AND LAND AT DARPAN COLONY, GWALIOR, MP Tender Invited by: Madhya Pradesh Road Development Corporation Dated: 23 February 2021 1 This page has been intentionally left blank 2 Table of Contents 1. NOTICE FOR INVITING TENDER CUM AUCTION..................................................... 5 1.1 Datasheet ......................................................................................................... 5 1.2 Details of the Property .................................................................................... 7 1.3 Tender Fee and Earnest Money Deposit (EMD) .............................................. 8 1.4 Bid Validity ....................................................................................................... 8 1.5 Eligible Entity ................................................................................................... 8 1.6 Pre-bid Meeting ............................................................................................... 8 1.7 Site Visit ........................................................................................................... 8 2. Instructions to Bidders ........................................................................................... 8 2.1 General ............................................................................................................ 9 2.2 Registration ...................................................................................................... 9 2.3 Searching for -
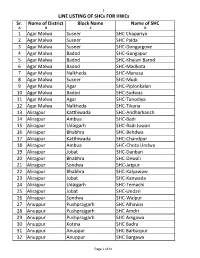
HWC-SHC Allotment List for CCCH
1 LINE LISTING OF SHCs FOR HWCs Sr. Name of District Block Name Name of SHC A B C D 1 Agar Malwa Susner SHC Chapariya 2 Agar Malwa Susner SHC Palda 3 Agar Malwa Susner SHC-Dongargove 4 Agar Malwa Badod SHC-Gangapur 5 Agar Malwa Badod SHC-Khajuri Barod 6 Agar Malwa Badod SHC-Madkota 7 Agar Malwa Nalkheda SHC-Manasa 8 Agar Malwa Susner SHC-Modi 9 Agar Malwa Agar SHC-Piplonkalan 10 Agar Malwa Badod SHC-Sudwas 11 Agar Malwa Agar SHC-Tanodiya 12 Agar Malwa Nalkheda SHC-Tikona 13 Alirajpur Katthiwada SHC-Andharkanch 14 Alirajpur Ambua SHC-Badi 15 Alirajpur Udaigarh SHC-Badi Juwari 16 Alirajpur Bhabhra SHC-Behdwa 17 Alirajpur Katthiwada SHC-Chandpur 18 Alirajpur Ambua SHC-Chota Undwa 19 Alirajpur Jobat SHC-Danbari 20 Alirajpur Bhabhra SHC-Dewali 21 Alirajpur Sondwa SHC-Jetpur 22 Alirajpur Bhabhra SHC-Kalyawaw 23 Alirajpur Jobat SHC-Kanwada 24 Alirajpur Udaigarh SHC-Temachi 25 Alirajpur Jobat SHC-Undari 26 Alirajpur Sondwa SHC-Walpur 27 Anuppur Pushprajgarh SHC Alhawar 28 Anuppur Pushprajgarh SHC Amdri 29 Anuppur Pushprajgarh SHC Amgawa 30 Anuppur Kotma SHC Badra 31 Anuppur Anuppur SHC Barbaspur 32 Anuppur Anuppur SHC Bargawa Page 1 of 41 2 LINE LISTING OF SHCs FOR HWCs Sr. Name of District Block Name Name of SHC 33 Anuppur Jaithari SHC Beend 34 Anuppur Kotma SHC Beiharbandh 35 Anuppur Pushprajgarh SHC Bilaspur 36 Anuppur Kotma SHC Chaka 37 Anuppur Jaithari SHC Choi 38 Anuppur Jaithari SHC Chorbhdhi 39 Anuppur Jaithari SHC Chulha 40 Anuppur Kotma SHC Dhangawa 41 Anuppur Pushprajgarh SHC Gonda 42 Anuppur Jaithari SHC Gorshi 43 Anuppur Anuppur -

Qualitative and Quantitative Evaluation of Water
J. Environ. Res. Develop. Journal of Environmental Research And Development Vol. 8 No. 3, January-March 2014 Review paper QUALITATIVE AND QUANTITATIVE EVALUATION OF WATER SOURCES OF FEW AREAS IN AND AROUND GWALIOR, MADHYA PRADESH, INDIA Saxena Richa* and Sharma Manju Department of Engineering Chemistry, ITM GOI, Gwalior, Madhya Pradesh (INDIA) Received September 25, 2013 Accepted January 09, 2014 ABSTRACT The present study is a review on the water analysis of some areas in Gwalior region conducted by various research groups in recent years. Various water sources of Gwalior region were assessed for its irrigational suitability, fish cultures well as drinking purpose. In this paper, different research studies are summarized on water analysis of different areas in and around Gwalior region, which is helpful to know the quality of water of this area and therefore its usage. Various physico-chemical parameters which were commonly tested by these groups are pH, conductivity, total hardness, TDS, turbidity, DO,several inorganic ions etc. The cumulative evidences obtained after analysing the water samples, supports the fact that the water of this region contains high pathogenic content, TDS, fluoride, chloride and sodium ion concentration mainly and in few samples high BOD and COD is indicative of the pollution status of these water sources. Thus, making it unfit for drinking purpose and requires proper treatement, however in some cases boiling and filtering of water before its usage would be suitable and for defluorination Nalgonda technique at domestic level is suggested. Key Words : pH, Conductivity, TDS, Turbidity, Nalgonda technique INTRODUCTION hydrochemical analysis underlies the fact that the chemistry of the ground water can directly Historically, Madhya Pradesh State used to be rated with the source of water, climate and have six regions. -
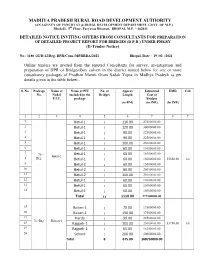
NIT-DPR-Bridges-3180
MADHYA PRADESH RURAL ROAD DEVELOPMENT AUTHORITY (AN AGENCY OF PANCHYAT & RURAL DEVELOPMENT DEPARTMENT, GOVT. OF M.P.) Block-II, 5th Floor, Paryavas Bhawan, BHOPAL M.P. – 462011 DETAILED NOTICE INVITING OFFERS FROM CONSULTANTS FOR PREPARATION OF DETAILED PROJECT REPORT FOR BRIDGES (D.P.R.) UNDER PMGSY (E-Tender Notice) No./ 3180 /22/D-12/Brg. DPR-Cons./MPRRDA/2021 Bhopal, Date : 19 /02 /2021 Online tenders are invited from the reputed Consultants for survey, investigation and preparation of DPR of Bridges/Box culvert in the district named below for one or more consultancy packages of Pradhan Mantri Gram Sadak Yojna in Madhya Pradesh as per details given in the table below:- S. No. Package Name of Name of PIU No. of Approx Estimated EMD Call No. Nodal included in the Bridges Length Cost of P.I.U. package Bridges (in RM) (in INR) (In INR) 1 2 3 4 5 6 7 8 9 `1 Betul-1 1 110.00 27500000.00 2 Betul-1 1 120.00 30000000.00 3 Betul-1 1 90.00 22500000.00 4 Betul-1 1 90.00 22500000.00 5 Betul-1 1 100.00 25000000.00 6 Betul-1 1 60.00 15000000.00 7 75 - Betul-1 1 60.00 15000000.00 Betul-2 8 Brg. Betul-1 1 60.00 15000000.00 55500.00 1st 9 Betul-2 1 60.00 15000000.00 10 Betul-2 1 80.00 20000000.00 11 Betul-2 1 100.00 25000000.00 12 Betul-1 1 60.00 15000000.00 13 Betul-1 1 60.00 15000000.00 14 Betul-1 1 60.00 15000000.00 Total 14 1110.00 277500000.00 15 Raisen-1 1 70.00 17500000.00 16 Raisen-1 1 150.00 37500000.00 17 Harda 1 90.00 22500000.00 76 -Brg. -

District Census Handbook, Gwalior, Part X(A)
CENSUS OF INDIA 1971 SERIES 10 MADHY A PRADESH DISTRICT CENSUS HANDBOOK PARTS X (A) & X(B) VILLAGE AND TOWN DIRECTORY VILLAGE AND TOWN-WISE PRIMARY CENSUS ABSTRACT OWALlOR DISTRICT A. K. PANDYA OF THE INDIAN ADMINISTRATIVE SBRVICE DIRECTOR OF CENSUS OPERATIONS. MADHYA PRADESH PUBLISHED BY TJlE GOVERNMENT OF MADHYA PRADESH 1977 1971 CENSUS PUBLICATIONS, MADHYA PRADESH (All the Census Publications of this State will bear series No. 10) PART I Census General Report including Subsidiary (in Sub-Parts) Tables PART II-A Census Tables on population PART II-B Economic Tables (in Sub-Parts) PART II-C Social and Cultural Tables (in Sub-Parts) PART III-A Establishment Report and Subsidiary Tables PART HI-B Establishment Tables PART IV Housing Report and Tables PART V Special Tables & Ethnographic Notes on (in Sub-Parts) Scheduled Castes & Scheduled Tribes PART VI-A Town Directory PART VI-B Special Survey Reports on selected towns PART VI-C Survey Reports on selected villages PART VII Special Report on Graduates and Technical Personnel PART VIII-A Administration Report-Enumeration PART VIII-B Administration Report-Tabulation PART IX Census Atlas PART IX-A Administrative Atlas STATE GOVERNMENT PUBLICATIONS PART X-A Village and Town Directory PART X-B Village and Town Primary Census Abstracts PART X-C Analytical Report and Administrative statements & District Census Tables - (District Census Handbooks are published under Part X in 3 Parts-A, B & C fo!_ each of the 43 di$lricts in tht' State, Parts A and B are published in one volume). CONTENTS Page 1. Preface i-ii 2.