Fossorial Origin of the Turtle Shell
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ontogeny and Distribution of Countershading in Colonies of the Naked Mole-Rat (Heterocephalus Glaber)
J. Zool., Lond. (2001) 253, 351±357 # 2001 The Zoological Society of London Printed in the United Kingdom The ontogeny and distribution of countershading in colonies of the naked mole-rat (Heterocephalus glaber) Stanton Braude1*, Deborah Ciszek2, Nancy E. Berg3 and Nancy Shefferly4 1 International Center for Tropical Ecology at UMSL and Washington University, St Louis, MO 63130, U.S.A. 2 University of Michigan, Ann Arbor, MI 48109-1079, U.S.A. 3 Washington University, St Louis, MO 63130, U.S.A. 4 Oakland Community College, Farmington Hills, MI 48334, U.S.A. (Accepted 22 February 2000) Abstract Most naked mole-rats Heterocephalus glaber are countershaded, with purple-grey dorsal but pale pink ventral skin. The exceptions to this coloration pattern are uniformly pink, and include newborn pups, most queens and breeding males, and very old animals. Countershading begins to appear at 2±3 weeks of age and begins to disappear at c. 7 years of age. Countershading may provide camou¯age when young naked mole-rats are above ground attempting to disperse. Therefore, reproductives and older workers may lose this coloration once they are unlikely to leave the burrow. Alternative hypotheses for pigmentation that we considered include: thermoregulation, and protection from abrasion or from damaging ultraviolet radiation. These hypotheses are not necessarily mutually exclusive, but do lead to different predictions regarding the development of pigmentation and which colony members should be countershaded. Key words: Heterocephalus glaber, naked mole-rat, countershading, adaptive coloration INTRODUCTION and tail, rows of brushes between the toes, and scattered bristles (Thigpen, 1940; Daly & Buffenstein, 1998). -

71St Annual Meeting Society of Vertebrate Paleontology Paris Las Vegas Las Vegas, Nevada, USA November 2 – 5, 2011 SESSION CONCURRENT SESSION CONCURRENT
ISSN 1937-2809 online Journal of Supplement to the November 2011 Vertebrate Paleontology Vertebrate Society of Vertebrate Paleontology Society of Vertebrate 71st Annual Meeting Paleontology Society of Vertebrate Las Vegas Paris Nevada, USA Las Vegas, November 2 – 5, 2011 Program and Abstracts Society of Vertebrate Paleontology 71st Annual Meeting Program and Abstracts COMMITTEE MEETING ROOM POSTER SESSION/ CONCURRENT CONCURRENT SESSION EXHIBITS SESSION COMMITTEE MEETING ROOMS AUCTION EVENT REGISTRATION, CONCURRENT MERCHANDISE SESSION LOUNGE, EDUCATION & OUTREACH SPEAKER READY COMMITTEE MEETING POSTER SESSION ROOM ROOM SOCIETY OF VERTEBRATE PALEONTOLOGY ABSTRACTS OF PAPERS SEVENTY-FIRST ANNUAL MEETING PARIS LAS VEGAS HOTEL LAS VEGAS, NV, USA NOVEMBER 2–5, 2011 HOST COMMITTEE Stephen Rowland, Co-Chair; Aubrey Bonde, Co-Chair; Joshua Bonde; David Elliott; Lee Hall; Jerry Harris; Andrew Milner; Eric Roberts EXECUTIVE COMMITTEE Philip Currie, President; Blaire Van Valkenburgh, Past President; Catherine Forster, Vice President; Christopher Bell, Secretary; Ted Vlamis, Treasurer; Julia Clarke, Member at Large; Kristina Curry Rogers, Member at Large; Lars Werdelin, Member at Large SYMPOSIUM CONVENORS Roger B.J. Benson, Richard J. Butler, Nadia B. Fröbisch, Hans C.E. Larsson, Mark A. Loewen, Philip D. Mannion, Jim I. Mead, Eric M. Roberts, Scott D. Sampson, Eric D. Scott, Kathleen Springer PROGRAM COMMITTEE Jonathan Bloch, Co-Chair; Anjali Goswami, Co-Chair; Jason Anderson; Paul Barrett; Brian Beatty; Kerin Claeson; Kristina Curry Rogers; Ted Daeschler; David Evans; David Fox; Nadia B. Fröbisch; Christian Kammerer; Johannes Müller; Emily Rayfield; William Sanders; Bruce Shockey; Mary Silcox; Michelle Stocker; Rebecca Terry November 2011—PROGRAM AND ABSTRACTS 1 Members and Friends of the Society of Vertebrate Paleontology, The Host Committee cordially welcomes you to the 71st Annual Meeting of the Society of Vertebrate Paleontology in Las Vegas. -

Morphology and Burrowing Energetics of Semi-Fossorial Skinks (Liopholis Spp.) Nicholas C
© 2015. Published by The Company of Biologists Ltd | The Journal of Experimental Biology (2015) 218, 2416-2426 doi:10.1242/jeb.113803 RESEARCH ARTICLE Morphology and burrowing energetics of semi-fossorial skinks (Liopholis spp.) Nicholas C. Wu1,*, Lesley A. Alton1, Christofer J. Clemente1, Michael R. Kearney2 and Craig R. White1 ABSTRACT mouse (Notomys alexis), can expend 5000 times more energy −1 −1 Burrowing is an important form of locomotion in reptiles, but no study burrowing than running (7.1 kJ m compared with 1.2 J m ; has examined the energetic cost of burrowing for reptiles. This is White et al., 2006b). Despite the considerable energetic cost, significant because burrowing is the most energetically expensive burrowing has many benefits. These include food storage, access to mode of locomotion undertaken by animals and many burrowing underground food, a secure micro-environment free from predators species therefore show specialisations for their subterranean lifestyle. and extreme environmental gradients (Robinson and Seely, 1980), We examined the effect of temperature and substrate characteristics nesting (Seymour and Ackerman, 1980), hibernation (Moberly, (coarse sand or fine sand) on the net energetic cost of burrowing 1963) and enhanced acoustics to facilitate communication (Bennet- (NCOB) and burrowing rate in two species of the Egernia group of Clark, 1987). skinks (Liopholis striata and Liopholis inornata) compared with other Animals utilise a range of methods to burrow through soil, burrowing animals. We further tested for morphological specialisations depending on soil characteristics (density, particle size and moisture among burrowing species by comparing the relationship between content) and body morphology (limbed or limbless). -

University of Florida Thesis Or Dissertation Formatting
UNDERSTANDING CARNIVORAN ECOMORPHOLOGY THROUGH DEEP TIME, WITH A CASE STUDY DURING THE CAT-GAP OF FLORIDA By SHARON ELIZABETH HOLTE A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2018 © 2018 Sharon Elizabeth Holte To Dr. Larry, thank you ACKNOWLEDGMENTS I would like to thank my family for encouraging me to pursue my interests. They have always believed in me and never doubted that I would reach my goals. I am eternally grateful to my mentors, Dr. Jim Mead and the late Dr. Larry Agenbroad, who have shaped me as a paleontologist and have provided me to the strength and knowledge to continue to grow as a scientist. I would like to thank my colleagues from the Florida Museum of Natural History who provided insight and open discussion on my research. In particular, I would like to thank Dr. Aldo Rincon for his help in researching procyonids. I am so grateful to Dr. Anne-Claire Fabre; without her understanding of R and knowledge of 3D morphometrics this project would have been an immense struggle. I would also to thank Rachel Short for the late-night work sessions and discussions. I am extremely grateful to my advisor Dr. David Steadman for his comments, feedback, and guidance through my time here at the University of Florida. I also thank my committee, Dr. Bruce MacFadden, Dr. Jon Bloch, Dr. Elizabeth Screaton, for their feedback and encouragement. I am grateful to the geosciences department at East Tennessee State University, the American Museum of Natural History, and the Museum of Comparative Zoology at Harvard for the loans of specimens. -

Ecological Roles and Conservation Challenges of Social, Burrowing
REVIEWS REVIEWS REVIEWS Ecological roles and conservation challenges 477 of social, burrowing, herbivorous mammals in the world’s grasslands Ana D Davidson1,2*, James K Detling3, and James H Brown1 The world’s grassland ecosystems are shaped in part by a key functional group of social, burrowing, herbivorous mammals. Through herbivory and ecosystem engineering they create distinctive and important habitats for many other species, thereby increasing biodiversity and habitat heterogeneity across the landscape. They also help maintain grassland presence and serve as important prey for many predators. However, these burrowing mammals are facing myriad threats, which have caused marked decreases in populations of the best-studied species, as well as cascading declines in dependent species and in grassland habitat. To prevent or mitigate such losses, we recommend that grasslands be managed to promote the compatibility of burrowing mammals with human activities. Here, we highlight the important and often overlooked ecological roles of these burrowing mammals, the threats they face, and future management efforts needed to enhance their populations and grass- land ecosystems. Front Ecol Environ 2012; 10(9): 477–486, doi:10.1890/110054 (published online 28 Sep 2012) rassland ecosystems worldwide are fundamentally Australia (Figure 1). Often living in colonies ranging Gshaped by an underappreciated but key functional from tens to thousands of individuals, these mammals col- group of social, semi-fossorial (adapted to burrowing and lectively transform grassland landscapes through their bur- living underground), herbivorous mammals (hereafter, rowing and feeding activity. By grouping together socially, burrowing mammals). Examples include not only the phy- they also create distinctive habitat patches that serve as logenetically similar species of prairie dogs of North areas of concentrated prey for many predators. -

Variability of the Parietal Foramen and the Evolution of the Pineal Eye in South African Permo-Triassic Eutheriodont Therapsids
The sixth sense in mammalian forerunners: Variability of the parietal foramen and the evolution of the pineal eye in South African Permo-Triassic eutheriodont therapsids JULIEN BENOIT, FERNANDO ABDALA, PAUL R. MANGER, and BRUCE S. RUBIDGE Benoit, J., Abdala, F., Manger, P.R., and Rubidge, B.S. 2016. The sixth sense in mammalian forerunners: Variability of the parietal foramen and the evolution of the pineal eye in South African Permo-Triassic eutheriodont therapsids. Acta Palaeontologica Polonica 61 (4): 777–789. In some extant ectotherms, the third eye (or pineal eye) is a photosensitive organ located in the parietal foramen on the midline of the skull roof. The pineal eye sends information regarding exposure to sunlight to the pineal complex, a region of the brain devoted to the regulation of body temperature, reproductive synchrony, and biological rhythms. The parietal foramen is absent in mammals but present in most of the closest extinct relatives of mammals, the Therapsida. A broad ranging survey of the occurrence and size of the parietal foramen in different South African therapsid taxa demonstrates that through time the parietal foramen tends, in a convergent manner, to become smaller and is absent more frequently in eutherocephalians (Akidnognathiidae, Whaitsiidae, and Baurioidea) and non-mammaliaform eucynodonts. Among the latter, the Probainognathia, the lineage leading to mammaliaforms, are the only one to achieve the complete loss of the parietal foramen. These results suggest a gradual and convergent loss of the photoreceptive function of the pineal organ and degeneration of the third eye. Given the role of the pineal organ to achieve fine-tuned thermoregulation in ecto- therms (i.e., “cold-blooded” vertebrates), the gradual loss of the parietal foramen through time in the Karoo stratigraphic succession may be correlated with the transition from a mesothermic metabolism to a high metabolic rate (endothermy) in mammalian ancestry. -

Coexisting Fossorial Species Differ in Their Emergence and Movement Patterns
Zoology 121 (2017): 49-55 DOI: 10.1016/j.zool.2016.12.003 Out of the ground: Coexisting fossorial species differ in their emergence and movement patterns Diana Székelya,b, Dan Cogălniceanua,*, Paul Székelya, Mathieu Denoëlb a Ovidius University Constanța, Faculty of Natural and Agricultural Sciences, Aleea Universității 1, corp B, Constanța, 900470, Romania b Laboratory of Fish and Amphibian Ethology, Behavioural Biology Unit, Freshwater and Oceanic Science Unit of Research (FOCUS), University of Liège, 22 Quai van Beneden, Liège, 4020, Belgium Abstract Understanding the way species with similar niches can coexist is a challenge in ecology. The niche partitioning hypothesis has received much support, positing that species can exploit available resources in different ways. In the case of secretive species, behavioural mechanisms of partitioning are still poorly understood. This is especially true for fossorial frogs because individuals hide underground by day and are active only during the night. We investigated the nocturnal activity and tested the niche partitioning hypothesis in two syntopic fossorial spadefoot toads (Pelobates fuscus and P. syriacus) by examining interspecific variation in emergence from the soil. We employed a night vision recording system combined with video-tracking analyses in a replicated laboratory setting to quantify individual movement patterns, a procedure that has not been used until now to observe terrestrial amphibians. Most individuals appeared on the surface every night and returned to their original burrow (about 60% of the times), or dug a new one around morning. There was a large temporal overlap between the two species. However, P. syriacus was significantly more active than P. -

A New Basal Ornithopod Dinosaur from the Lower Cretaceous of China
A new basal ornithopod dinosaur from the Lower Cretaceous of China Yuqing Yang1,2,3, Wenhao Wu4,5, Paul-Emile Dieudonné6 and Pascal Godefroit7 1 College of Resources and Civil Engineering, Northeastern University, Shenyang, Liaoning, China 2 College of Paleontology, Shenyang Normal University, Shenyang, Liaoning, China 3 Key Laboratory for Evolution of Past Life and Change of Environment, Province of Liaoning, Shenyang Normal University, Shenyang, Liaoning, China 4 Key Laboratory for Evolution of Past Life and Environment in Northeast Asia, Ministry of Education, Jilin University, Changchun, Jilin, China 5 Research Center of Paleontology and Stratigraphy, Jilin University, Changchun, Jilin, China 6 Instituto de Investigación en Paleobiología y Geología, CONICET, Universidad Nacional de Río Negro, Rio Negro, Argentina 7 Directorate ‘Earth and History of Life’, Royal Belgian Institute of Natural Sciences, Brussels, Belgium ABSTRACT A new basal ornithopod dinosaur, based on two nearly complete articulated skeletons, is reported from the Lujiatun Beds (Yixian Fm, Lower Cretaceous) of western Liaoning Province (China). Some of the diagnostic features of Changmiania liaoningensis nov. gen., nov. sp. are tentatively interpreted as adaptations to a fossorial behavior, including: fused premaxillae; nasal laterally expanded, overhanging the maxilla; shortened neck formed by only six cervical vertebrae; neural spines of the sacral vertebrae completely fused together, forming a craniocaudally-elongated continuous bar; fused scapulocoracoid with prominent -

Ecological Shifts in Sympatry: Kalahari Fossorial Lizards (Typhlosaurus)
Ecology (1974) 55: pp. 304-316 ECOLOGICAL SHIFTS IN SYMPATRY: KALAHARI FOSSORIAL LIZARDS (TYPHLOSA URUS)l RAYMOND B. HUEY,2 ERIC R. PIANKA, MICHAEL E. EGAN, AND LARRY W. COONS Department of Zoology, University of Texas, Austin, Texas 78712 Abstract. Two species of legless, fossorial skinks (Typhlosaurus) are partially sympatric in the Kalahari Desert. Typhlosaurus lineatus occur both in the sandridge and flatland geographic provinces of the Kalahari, in dune streets and on sandridges microgeographically in sympatry, and primarily under log and leaf litter microhabitats. Typhlosaurus gariepensis, always sympatric with T. lineatus, occur only in sandridge areas geographically, on sand- ridges microgeographically, and primarily in basal roots of bunch grass microhabitats. Micro- habitat overlap in sympatry is low. Morphologically, T. lineatus differ from T. gariepensis in being longer and in having a longer and wider head. Snout-vent lengths, head dimensions, and proportional head lengths of sympatric T. lineatus are larger than those of allopatric T. lineatus; thus T. lineatus displace from T. gariepensis in sympatry. Both species are viviparous, have one brood per year, and give birth in summer after a 5-mo gestation period. Maturity is reached at minimum ages of one and two-thirds yr. Mean litter size of T. lineatus is 1.6, whereas T. gariepensis have but one young. A response to competition between the two species is suggested by the facts that offspring of sympatric T. lineatus are significantly heavier than those of allopatric females, and that fewer sympatric T. lineatus females are reproductive than allopatric females. Because Typhlosaurus are termite specialists (92.4% of diet by volume), termites in guts were identified to species and caste. -

Burrows of Semi-Fossorial Vertebrates in Upland Communities of Central Florida: Their Architecture, Dispersion and Ecological Consequences
BURROWS OF SEMI-FOSSORIAL VERTEBRATES IN UPLAND COMMUNITIES OF CENTRAL FLORIDA: THEIR ARCHITECTURE, DISPERSION AND ECOLOGICAL CONSEQUENCES By ALTON EMORY KINLAW A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2006 1 To my daughter Skye Gabrielle Kinlaw, who has accomplished more in her short 11 years than most adults do in a lifetime. 2 ACKNOWLEDGMENTS I owe a major professional and personal debt to my chair, Michael Moulton for his guidance. In particular, at a time when I was discouraged due to health and financial reasons, he encouraged me to stay the course. Because of his quantitative background in orthithology, herpetology, and mammalogy, he has a unique intellectual perspective about vertebrate ecology and always challenged me to ask the right questions. Additionally, he provided me with a balance, Sherman live traps, and microscope. My co-chair, Dick Franz, shared his comprehensive knowledge about natural history of Florida fauna, and served as project director of the Avon Park project issues. He encouraged me to write the Avon vertebrate diversity chapter and carefully critiqued all of my papers. My research is based upon the theoretical background developed by Jim Nichols and his colleagues at Patuxent Wildlife Research Center. Jerry Butler, UF Department of Entomology and Nematology, provided sticky traps and “Tick Sucker” vacuums, reprints, and freely shared his extensive knowledge of insect fauna that use tortoise burrows. Ken Portier graciously stayed on my committee after leaving UF for a position in Atlanta, and allowed me to visit him with my questions. -

Jaw Shape and Mechanical Advantage Are Indicative of Diet in Mesozoic Mammals ✉ Nuria Melisa Morales-García 1 , Pamela G
ARTICLE https://doi.org/10.1038/s42003-021-01757-3 OPEN Jaw shape and mechanical advantage are indicative of diet in Mesozoic mammals ✉ Nuria Melisa Morales-García 1 , Pamela G. Gill1,2, Christine M. Janis 1,3 & Emily J. Rayfield 1 Jaw morphology is closely linked to both diet and biomechanical performance, and jaws are one of the most common Mesozoic mammal fossil elements. Knowledge of the dietary and functional diversity of early mammals informs on the ecological structure of palaeo- communities throughout the longest era of mammalian evolution: the Mesozoic. Here, we analyse how jaw shape and mechanical advantage of the masseter (MAM) and temporalis (MAT) muscles relate to diet in 70 extant and 45 extinct mammals spanning the Late 1234567890():,; Triassic-Late Cretaceous. In extant mammals, jaw shape discriminates well between dietary groups: insectivores have long jaws, carnivores intermediate to short jaws, and herbivores have short jaws. Insectivores have low MAM and MAT, carnivores have low MAM and high MAT, and herbivores have high MAM and MAT. These traits are also informative of diet among Mesozoic mammals (based on previous independent determinations of diet) and set the basis for future ecomorphological studies. 1 School of Earth Sciences, Wills Memorial Building, University of Bristol, Bristol, UK. 2 Department of Earth Sciences, Natural History Museum, London, UK. ✉ 3 Department of Ecology and Evolutionary Biology, Brown University, Providence, RI, USA. email: [email protected] COMMUNICATIONS BIOLOGY | (2021) 4:242 | https://doi.org/10.1038/s42003-021-01757-3 | www.nature.com/commsbio 1 ARTICLE COMMUNICATIONS BIOLOGY | https://doi.org/10.1038/s42003-021-01757-3 ur understanding of Mesozoic mammals has dramatically metric) has been used as a proxy for prey choice and feeding improved in the past three decades. -
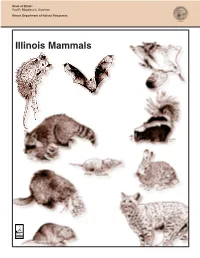
Illinois Mammals What Are Mammals?
State of Illinois Rod R. Blagojevich, Governor Illinois Department of Natural Resources Illinois Mammals What are mammals? There are many types of animals in Illinois. Find the Mammals. Insects, spiders, fishes, frogs and birds are Five of the animals shown below are just some of the animals you can find in our mammals. Circle the five mammals. state. Mammals are animals, too. How can you tell if an animal is a mammal? ª A mammal has four limbs (arms/legs). ª It has hair or fur. ª It is warm-blooded (its body temperature is kept at the same level regardless of the outside temperature). ª Most mammals have young born after developing inside the mother's body in a special organ called a uterus. A few mammal species lay eggs, but these mammals do not live in Illinois. ª After birth, the young are fed with milk produced in the female’s mammary glands. ª A mammal has a large and complex brain. Using this Activity Book: For the Educator When using this activity book, students will become familiar with the traits and appearance of 16 mammal species that live in our state. The information and activities can help you meet the following Illinois Learning Standards: 6.B.1; 6.B.2; 6.C.1; 6.C.2; 10.A.1a; 10.A.1b; 10.A.2a; 10.A.2b; 12.B.2a; 12.B.2b; 12.B.3b. You will find that the Biodiversity of Illinois series of CD-ROMs from the Illinois Department of Natural Resources (IDNR) supplements this booklet by providing sounds, photographs and other information about the species.