Chromosomal Microarray Analysis (CMA)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moderate the MAOA-L Allele Expression with CRISPR/Cas9 System
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 23 April 2018 doi:10.20944/preprints201804.0275.v1 1 Review 2 Moderate the MAOA-L Allele Expression with CRISPR/Cas9 System 3 Martin L. Nelwan 4 Department of Animal Science – Other 5 Nelwan Institution for Human Resource Development 6 Jl. A. Yani No. 24 7 Palu, Sulawesi Tengah, Indonesia 8 Email: [email protected] 9 Abstract: Antisocial behavior is a behavior disorder inherited according to the inheritance of X-linked 10 chromosome. Mutations in the MAOA gene can cause different behaviors in humans. These can comprise 11 violent behavior or antisocial behavior. Low MAOA (MAOA-L) allele activity can cause antisocial 12 behavior in both healthy and unhealthy people. Antisocial from healthy males can originate from 13 maltreatment during childhood. There are no drugs for the treatment of antisocial behavior permanently 14 at this time. MAOA inhibitor can reverse antisocial behavior in animal models. To cure antisocial 15 behavior in the future, the CRISPR/Cas9 system in combination with iPSCs or ssODN methods for 16 instance can be used. This system has succeeded to correct erroneous segments in the F8 gene and F9 17 gene. Both genes occupy the X chromosome. The MAOA gene also occupies the X chromosome. It seems 18 that CRISPR/Cas9 system may be a beneficial tool to edit erroneous segments in the MAOA gene to treat 19 antisocial behavior. 20 Keywords: advanced therapy, aggressive, antisocial, behavior, MAOA. 21 22 1. Introduction 23 Antisocial behavior is a hereditary disorder inherited through an X-linked recessive inheritance 24 pattern. -

Genes in Eyecare Geneseyedoc 3 W.M
Genes in Eyecare geneseyedoc 3 W.M. Lyle and T.D. Williams 15 Mar 04 This information has been gathered from several sources; however, the principal source is V. A. McKusick’s Mendelian Inheritance in Man on CD-ROM. Baltimore, Johns Hopkins University Press, 1998. Other sources include McKusick’s, Mendelian Inheritance in Man. Catalogs of Human Genes and Genetic Disorders. Baltimore. Johns Hopkins University Press 1998 (12th edition). http://www.ncbi.nlm.nih.gov/Omim See also S.P.Daiger, L.S. Sullivan, and B.J.F. Rossiter Ret Net http://www.sph.uth.tmc.edu/Retnet disease.htm/. Also E.I. Traboulsi’s, Genetic Diseases of the Eye, New York, Oxford University Press, 1998. And Genetics in Primary Eyecare and Clinical Medicine by M.R. Seashore and R.S.Wappner, Appleton and Lange 1996. M. Ridley’s book Genome published in 2000 by Perennial provides additional information. Ridley estimates that we have 60,000 to 80,000 genes. See also R.M. Henig’s book The Monk in the Garden: The Lost and Found Genius of Gregor Mendel, published by Houghton Mifflin in 2001 which tells about the Father of Genetics. The 3rd edition of F. H. Roy’s book Ocular Syndromes and Systemic Diseases published by Lippincott Williams & Wilkins in 2002 facilitates differential diagnosis. Additional information is provided in D. Pavan-Langston’s Manual of Ocular Diagnosis and Therapy (5th edition) published by Lippincott Williams & Wilkins in 2002. M.A. Foote wrote Basic Human Genetics for Medical Writers in the AMWA Journal 2002;17:7-17. A compilation such as this might suggest that one gene = one disease. -

Genetic and Developmental Basis of Renal Coloboma (Papillorenal) Syndrome
Perspective Genetic and developmental basis of renal coloboma (papillorenal) syndrome Expert Rev. Ophthalmol. 4(2), 135–xxx (2009) Lisa A Schimmenti Renal coloboma syndrome, also known as papillorenal syndrome, is characterized by optic Department of Pediatrics and nerve anomalies and kidney hypodysplasia. Autosomal dominant mutations in the gene Ophthalmology, University of encoding the paired box transcription factor PAX2 can be identified in nearly half of all Minnesota Medical School, 420 patients with this phenotype. The primary ophthalmologic findings include congenital central Delaware Street Southeast, retinal vasculature absence associated with abnormalities in retinal blood vessel patterning MMC 730, Minnesota, and deeply excavated optic discs. Other published findings include optic nerve hypoplasia, MN 55455, USA optic nerve cyst, optic nerve pits, retinal coloboma, microphthalmia and scleral staphyloma. Tel.: +1 612 624 5613 Visual acuity ranges from normal to severe impairment. Up to one third of affected patients Fax: +1 612 626 2993 will develop end-stage renal disease. Mouse and zebrafish withPax2/pax2a mutations provide [email protected] developmentally based explanations for the observed phenotypic observations in affected patients. KEYWORDS: optic nerve coloboma • optic nerve dysplasia • papillorenal syndrome • PAX2 • renal coloboma syndrome The clinical presentation of optic nerve anomalies total of 10 years later, Weaver et al. reported a case associated with renal hypodysplasia should alert the of two brothers, both with optic nerve colobomas, clinician to the possibility that a patient may have interstitial nephritis and renal failure [3]. Weaver renal coloboma syndrome, a condition also known noted the similarity of the optic nerve colobomas as papillorenal syndrome (OMIM#120330). The in his patients with the ‘morning glory anomaly’, optic nerve findings could be described as a ‘dys- previously reported by Karcher et al. -

Ophthalmology
Ophthalmology Information for health professionals MEDICAL GENETIC TESTING FOR OPHTHALMOLOGY Recent technologies, in particularly Next Generation Sequencing (NGS), allows fast, accurate and valuable diagnostic tests. For Ophthalmology, CGC Genetics has an extensive list of medical genetic tests with clinical integration of results by our Medical Geneticists. 1. EXOME SEQUENCING: Exome Sequencing is a very efficient strategy to study most exons of a patient’s genome, unraveling mutations associated with specific disorders or phenotypes. With this diagnostic strategy, patients can be studied with a significantly reduced turnaround time and cost. CGC Genetics has available 2 options for Exome Sequencing: • Whole Exome Sequencing (WES), which analyzes the entire exome (about 20 000 genes); • Disease Exome by CGC Genetics, which analyzes about 6 000 clinically-relevant genes. Any of these can be performed in the index case or in a Trio. 2. NGS PANELS For NGS panels, several genes associated with the same phenotype are simultaneously sequenced. These panels provide increased diagnostic capability with a significantly reduced turnaround time and cost. CGC Genetics has several NGS panels for Ophthalmology that are constantly updated (www.cgcgenetics.com). Any gene studied in exome or NGS panel can also be individually sequenced and analyzed for deletion/duplication events. 3. EXPERTISE IN MEDICAL GENETICS CGC Genetics has Medical Geneticists specialized in genetic counseling for ophthalmological diseases who may advice in choosing the most appropriate -

WES Gene Package Multiple Congenital Anomalie.Xlsx
Whole Exome Sequencing Gene package Multiple congenital anomalie, version 5, 1‐2‐2018 Technical information DNA was enriched using Agilent SureSelect Clinical Research Exome V2 capture and paired‐end sequenced on the Illumina platform (outsourced). The aim is to obtain 8.1 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA‐MEM algorithm (reference: http://bio‐bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A4GALT [Blood group, P1Pk system, P(2) phenotype], 111400 607922 101 100 100 99 [Blood group, P1Pk system, p phenotype], 111400 NOR polyagglutination syndrome, 111400 AAAS Achalasia‐addisonianism‐alacrimia syndrome, 231550 605378 73 100 100 100 AAGAB Keratoderma, palmoplantar, -

Whole Exome Sequencing Gene Package Intellectual Disability, Version 9.1, 31-1-2020
Whole Exome Sequencing Gene package Intellectual disability, version 9.1, 31-1-2020 Technical information DNA was enriched using Agilent SureSelect DNA + SureSelect OneSeq 300kb CNV Backbone + Human All Exon V7 capture and paired-end sequenced on the Illumina platform (outsourced). The aim is to obtain 10 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate and non-unique reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA-MEM algorithm (reference: http://bio-bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A2ML1 {Otitis media, susceptibility to}, 166760 610627 66 100 100 96 AARS1 Charcot-Marie-Tooth disease, axonal, type 2N, 613287 601065 63 100 97 90 Epileptic encephalopathy, early infantile, 29, 616339 AASS Hyperlysinemia, -
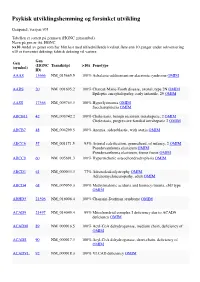
Psykisk Utviklingshemming Og Forsinket Utvikling
Psykisk utviklingshemming og forsinket utvikling Genpanel, versjon v03 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC >x10 Andel av genet som har blitt lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering x10 er forventet dekning; faktisk dekning vil variere. Gen Gen (HGNC Transkript >10x Fenotype (symbol) ID) AAAS 13666 NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AARS 20 NM_001605.2 100% Charcot-Marie-Tooth disease, axonal, type 2N OMIM Epileptic encephalopathy, early infantile, 29 OMIM AASS 17366 NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCB11 42 NM_003742.2 100% Cholestasis, benign recurrent intrahepatic, 2 OMIM Cholestasis, progressive familial intrahepatic 2 OMIM ABCB7 48 NM_004299.5 100% Anemia, sideroblastic, with ataxia OMIM ABCC6 57 NM_001171.5 93% Arterial calcification, generalized, of infancy, 2 OMIM Pseudoxanthoma elasticum OMIM Pseudoxanthoma elasticum, forme fruste OMIM ABCC9 60 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 61 NM_000033.3 77% Adrenoleukodystrophy OMIM Adrenomyeloneuropathy, adult OMIM ABCD4 68 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 21396 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 21497 NM_014049.4 99% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM 89 NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS 90 NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL 92 NM_000018.3 100% VLCAD -

Test Catalogue August 2019
Test Catalogue August 2019 www.centogene.com/catalogue Table of Contents CENTOGENE CLINICAL DIAGNOSTIC PRODUCTS AND SERVICES › Whole Exome Testing 4 › Whole Genome Testing 5 › Genome wide CNV Analysis 5 › Somatic Mutation Analyses 5 › Biomarker Testing, Biochemical Testing 6 › Prenatal Testing 7 › Additional Services 7 › Metabolic Diseases 9 - 21 › Neurological Diseases 23 - 47 › Ophthalmological Diseases 49 - 55 › Ear, Nose and Throat Diseases 57 - 61 › Bone, Skin and Immune Diseases 63 - 73 › Cardiological Diseases 75 - 79 › Vascular Diseases 81 - 82 › Liver, Kidney and Endocrinological Diseases 83 - 89 › Reproductive Genetics 91 › Haematological Diseases 93 - 96 › Malformation and/or Retardation Syndromes 97 - 107 › Oncogenetics 109 - 113 ® › CentoXome - Sequencing targeting exonic regions of ~20.000 genes Test Test name Description code CentoXome® Solo Medical interpretation/report of WES findings for index 50029 CentoXome® Solo - Variants Raw data; fastQ, BAM, Vcf files along with variant annotated file in xls format for index 50028 CentoXome® Solo - with CNV Medical interpretation/report of WES including CNV findings for index 50103 Medical interpretation/report of WES in index, package including genome wide analyses of structural/ CentoXome® Solo - with sWGS 50104 large CNVs through sWGS Medical interpretation/report of WES in index, package including genome wide analyses of structural/ CentoXome® Solo - with aCGH 750k 50122 large CNVs through 750k microarray Medical interpretation/report of WES in index, package including genome -

(12) Patent Application Publication (10) Pub. No.: US 2016/0281166 A1 BHATTACHARJEE Et Al
US 20160281 166A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0281166 A1 BHATTACHARJEE et al. (43) Pub. Date: Sep. 29, 2016 (54) METHODS AND SYSTEMIS FOR SCREENING Publication Classification DISEASES IN SUBJECTS (51) Int. Cl. (71) Applicant: PARABASE GENOMICS, INC., CI2O I/68 (2006.01) Boston, MA (US) C40B 30/02 (2006.01) (72) Inventors: Arindam BHATTACHARJEE, G06F 9/22 (2006.01) Andover, MA (US); Tanya (52) U.S. Cl. SOKOLSKY, Cambridge, MA (US); CPC ............. CI2O 1/6883 (2013.01); G06F 19/22 Edwin NAYLOR, Mt. Pleasant, SC (2013.01); C40B 30/02 (2013.01); C12O (US); Richard B. PARAD, Newton, 2600/156 (2013.01); C12O 2600/158 MA (US); Evan MAUCELI, (2013.01) Roslindale, MA (US) (21) Appl. No.: 15/078,579 (57) ABSTRACT (22) Filed: Mar. 23, 2016 Related U.S. Application Data The present disclosure provides systems, devices, and meth (60) Provisional application No. 62/136,836, filed on Mar. ods for a fast-turnaround, minimally invasive, and/or cost 23, 2015, provisional application No. 62/137,745, effective assay for Screening diseases, such as genetic dis filed on Mar. 24, 2015. orders and/or pathogens, in Subjects. Patent Application Publication Sep. 29, 2016 Sheet 1 of 23 US 2016/0281166 A1 SSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSS S{}}\\93? sau36 Patent Application Publication Sep. 29, 2016 Sheet 2 of 23 US 2016/0281166 A1 &**** ? ???zzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzz??º & %&&zzzzzzzzzzzzzzzzzzzzzzz &Sssssssssssssssssssssssssssssssssssssssssssssssssssssssss & s s sS ------------------------------ Patent Application Publication Sep. 29, 2016 Sheet 3 of 23 US 2016/0281166 A1 23 25 20 FG, 2. Patent Application Publication Sep. 29, 2016 Sheet 4 of 23 US 2016/0281166 A1 : S Patent Application Publication Sep. -

Use of Next Generation Sequencing for Isolated and Syndromic Anophthalmia, Microphthalmia and Coloboma (MAC): a New Approach to Molecular Genetic Diagnosis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archivio della ricerca- Università di Roma La Sapienza Use of Next Generation Sequencing for isolated and syndromic Anophthalmia, Microphthalmia and Coloboma (MAC): a new approach to molecular genetic diagnosis Department of Cellular Biotechnologies and Hematology PhD in Human Biology and Medical Genetics XXX° cycle 2014-2017 Candidate Dr. Iapichino Giuseppe 1235269 Supervisor Correlator Prof. Pizzuti Antonio Prof. Novelli Antonio A/A 2016/2017 1. INTRODUCTION 1.1 Eye embryogenesis 1.2 MAC: Mixed group of diseases 1.2.1 Microphthalmia, Anophthalmia and Coloboma 1.2.2 Syndromic diseases: CHARGE syndrome Cornelia de Lange syndrome Axenfeld-Rieger syndrome Lenz’s Microphtalmia Papillorenal syndrome 1.3 Molecular Diagnosis: Next Generation Sequencing 2. AIM OF THE STUDY 3. MATERIALS AND METHODS 3.1 Patients Recruitment 3.2 Molecular Analysis 3.2.1 Blood collection, DNA extraction and quantification 3.2.2 NGS panel construction: Design Studio 3.2.3 NextSeq-500: features and workflow Libraries preparation: Nextera Rapid Capture Custom Enrichment Cluster generation Sequencing 3.2.4 Sanger Sequencing 3.2.5 Data Analysis 4. RESULTS 5. DISCUSSION 6. CONCLUSION 7. BIBLIOGRAPHY 1. INTRODUCTION 1.1 The eye: embryogenesis Eye development begins around the 4th week of gestation and can be summarized in four main stages [Zagozewski J.L. et al.; 2014]: Optic vesicle formation Induction of crystalline lens Retinal tissue formation Optic fissure closure Each of these events is highly regulated and coordinated by various transcription factors and circulating molecules [Adler R. et al.; 2007]. -

Absence of Mutations in PAX6 Gene in Three Cases of Morning Glory Syndrome Associated with Isolated Growth Hormone Defi Ciency
Absence of Mutations in PAX6 Gene in Three Cases of Morning Glory Syndrome Associated with Isolated Growth Hormone Defi ciency ABSTRACT clinical case report Morning glory syndrome (MGS) is a congenital optic disc dysplasia often as- sociated with craniofacial anomalies, especially basal encephalocele and hy- popituitarism. Clinical signs are varied and often occult. The PAX6 gene is involved in ocular morphogenesis and is expressed in numerous ocular tissues during development especially in the developing central nervous system. The GIL GUERRA-JUNIOR aim of the present study is to evaluate PAX6 in MGS associated with isolated ANGELA MARIA SPINOLA-CASTRO growth hormone defi ciency. Three pre-pubertal males (A, B and C) with MGS and short stature due to growth hormone defi ciency, treated with recombinant ADRIANA A. SIVIERO-MIACHON human growth hormone with limited response, were reported. Two of them ROBERTO GOMES NOGUEIRA had basal encephalocele. Coding and non-coding sequences corresponding of SOFIA HELENA V. L EMOS-MARINI PAX6 different transcripts were analyzed by direct sequencing. Nucleotide vari- ations causing putative aminoacid change were not observed. Patient A pre- LILIA FREIRE RODRIGUES D’SOUZA-LI sented the new IVS2+9G>A transition, whereas patients A and C were PRISCILA CRISTINA DA SILVA heterozygous for known single nucleotide polymorphisms (SNP) within the in- EMERSON SALVADOR S. FRANÇA tron 4. In addition, two SNP heterozygoses were observed for patient C in both intron 9 and 13. Sequencing also revealed several nucleotide variations in pa- FERNANDA CAROLINE SOARDI tient B. Two heterozygoses for known polymorphisms were identifi ed along MARICILDA PALANDI DE MELLO with a novel C>A nucleotide change in intron 4. -

Prenatalscreen® Standard Technical Report
About PrenatalScreen® Prenatal Test PrenatalScreen® Prenatal Test is a genetic test that analyses fetal DNA, obtained from CVS or amniotic fluid following an invasive prenatal diagnosis, to screen for monogenic disorders in the fetus. Using the latest technologies, including Next Generation Sequencing (NGS), PrenatalScreen® Prenatal Test screen 744 genes for mutations causing over 1.000 severe genetic disorders in the fetus. PrenatalScreen® Prenatal Test allows for a comprehensive care and enables patients to make more informed reproductive decisions. Offering PrenatalScreen® Prenatal Test to a patient during pregnancy allows her to gain more knowledge about the potential to pass along a condition to the fetus. Aim of the test PrenatalScreen® Prenatal Test analyses DNA extracted from fetal cells in the amniotic fluid, collected through amniocentesis, or in the chorionic villi through villocentesis (CVS). The aim of this diagnositc test is to assess severe genetic diseases in the fetus, including the most common diseases in the European population. Genes listed in Table 1 were selected according to the incidence in the population of the disease caused by mutations in such genes, the severity of the clinical phenotype at birth and the importance of the related pathogenetic picture, in accordance with the indications of the American College of Medical Genetics (ACMG)(Grody et al., Genet Med 2013:15:482–483). PrenatalScreen®: Indication for testing PrenatalScreen® Prenatal Test is intended for patients who meet any of the following criteria: • Personal/familial anamnesis of hereditary genetic diseases; • For expectant mothers wishing to reduce the risk of a genetic diseases in the fetus; • For natural or in vitro fertilization (IVF)-derived pregnancies: • For couples using heterologus IVF procedures (egg/sperm donors).