Nyresykdommer V01
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genes in Eyecare Geneseyedoc 3 W.M
Genes in Eyecare geneseyedoc 3 W.M. Lyle and T.D. Williams 15 Mar 04 This information has been gathered from several sources; however, the principal source is V. A. McKusick’s Mendelian Inheritance in Man on CD-ROM. Baltimore, Johns Hopkins University Press, 1998. Other sources include McKusick’s, Mendelian Inheritance in Man. Catalogs of Human Genes and Genetic Disorders. Baltimore. Johns Hopkins University Press 1998 (12th edition). http://www.ncbi.nlm.nih.gov/Omim See also S.P.Daiger, L.S. Sullivan, and B.J.F. Rossiter Ret Net http://www.sph.uth.tmc.edu/Retnet disease.htm/. Also E.I. Traboulsi’s, Genetic Diseases of the Eye, New York, Oxford University Press, 1998. And Genetics in Primary Eyecare and Clinical Medicine by M.R. Seashore and R.S.Wappner, Appleton and Lange 1996. M. Ridley’s book Genome published in 2000 by Perennial provides additional information. Ridley estimates that we have 60,000 to 80,000 genes. See also R.M. Henig’s book The Monk in the Garden: The Lost and Found Genius of Gregor Mendel, published by Houghton Mifflin in 2001 which tells about the Father of Genetics. The 3rd edition of F. H. Roy’s book Ocular Syndromes and Systemic Diseases published by Lippincott Williams & Wilkins in 2002 facilitates differential diagnosis. Additional information is provided in D. Pavan-Langston’s Manual of Ocular Diagnosis and Therapy (5th edition) published by Lippincott Williams & Wilkins in 2002. M.A. Foote wrote Basic Human Genetics for Medical Writers in the AMWA Journal 2002;17:7-17. A compilation such as this might suggest that one gene = one disease. -

Genetic and Developmental Basis of Renal Coloboma (Papillorenal) Syndrome
Perspective Genetic and developmental basis of renal coloboma (papillorenal) syndrome Expert Rev. Ophthalmol. 4(2), 135–xxx (2009) Lisa A Schimmenti Renal coloboma syndrome, also known as papillorenal syndrome, is characterized by optic Department of Pediatrics and nerve anomalies and kidney hypodysplasia. Autosomal dominant mutations in the gene Ophthalmology, University of encoding the paired box transcription factor PAX2 can be identified in nearly half of all Minnesota Medical School, 420 patients with this phenotype. The primary ophthalmologic findings include congenital central Delaware Street Southeast, retinal vasculature absence associated with abnormalities in retinal blood vessel patterning MMC 730, Minnesota, and deeply excavated optic discs. Other published findings include optic nerve hypoplasia, MN 55455, USA optic nerve cyst, optic nerve pits, retinal coloboma, microphthalmia and scleral staphyloma. Tel.: +1 612 624 5613 Visual acuity ranges from normal to severe impairment. Up to one third of affected patients Fax: +1 612 626 2993 will develop end-stage renal disease. Mouse and zebrafish withPax2/pax2a mutations provide [email protected] developmentally based explanations for the observed phenotypic observations in affected patients. KEYWORDS: optic nerve coloboma • optic nerve dysplasia • papillorenal syndrome • PAX2 • renal coloboma syndrome The clinical presentation of optic nerve anomalies total of 10 years later, Weaver et al. reported a case associated with renal hypodysplasia should alert the of two brothers, both with optic nerve colobomas, clinician to the possibility that a patient may have interstitial nephritis and renal failure [3]. Weaver renal coloboma syndrome, a condition also known noted the similarity of the optic nerve colobomas as papillorenal syndrome (OMIM#120330). The in his patients with the ‘morning glory anomaly’, optic nerve findings could be described as a ‘dys- previously reported by Karcher et al. -

Ophthalmology
Ophthalmology Information for health professionals MEDICAL GENETIC TESTING FOR OPHTHALMOLOGY Recent technologies, in particularly Next Generation Sequencing (NGS), allows fast, accurate and valuable diagnostic tests. For Ophthalmology, CGC Genetics has an extensive list of medical genetic tests with clinical integration of results by our Medical Geneticists. 1. EXOME SEQUENCING: Exome Sequencing is a very efficient strategy to study most exons of a patient’s genome, unraveling mutations associated with specific disorders or phenotypes. With this diagnostic strategy, patients can be studied with a significantly reduced turnaround time and cost. CGC Genetics has available 2 options for Exome Sequencing: • Whole Exome Sequencing (WES), which analyzes the entire exome (about 20 000 genes); • Disease Exome by CGC Genetics, which analyzes about 6 000 clinically-relevant genes. Any of these can be performed in the index case or in a Trio. 2. NGS PANELS For NGS panels, several genes associated with the same phenotype are simultaneously sequenced. These panels provide increased diagnostic capability with a significantly reduced turnaround time and cost. CGC Genetics has several NGS panels for Ophthalmology that are constantly updated (www.cgcgenetics.com). Any gene studied in exome or NGS panel can also be individually sequenced and analyzed for deletion/duplication events. 3. EXPERTISE IN MEDICAL GENETICS CGC Genetics has Medical Geneticists specialized in genetic counseling for ophthalmological diseases who may advice in choosing the most appropriate -

American Board of Psychiatry and Neurology, Inc
AMERICAN BOARD OF PSYCHIATRY AND NEUROLOGY, INC. CERTIFICATION EXAMINATION IN NEUROLOGY 2015 Content Blueprint (January 13, 2015) Part A Basic neuroscience Number of questions: 120 01. Neuroanatomy 3-5% 02. Neuropathology 3-5% 03. Neurochemistry 2-4% 04. Neurophysiology 5-7% 05. Neuroimmunology/neuroinfectious disease 2-4% 06. Neurogenetics/molecular neurology, neuroepidemiology 2-4% 07. Neuroendocrinology 1-2% 08. Neuropharmacology 4-6% Part B Behavioral neurology, cognition, and psychiatry Number of questions: 80 01. Development through the life cycle 3-5% 02. Psychiatric and psychological principles 1-3% 03. Diagnostic procedures 1-3% 04. Clinical and therapeutic aspects of psychiatric disorders 5-7% 05. Clinical and therapeutic aspects of behavioral neurology 5-7% Part C Clinical neurology (adult and child) The clinical neurology section of the Neurology Certification Examination is comprised of 60% adult neurology questions and 40% child neurology questions. Number of questions: 200 01. Headache disorders 1-3% 02. Pain disorders 1-3% 03. Epilepsy and episodic disorders 1-3% 04. Sleep disorders 1-3% 05. Genetic disorders 1-3% 2015 ABPN Content Specifications Page 1 of 22 Posted: Certification in Neurology AMERICAN BOARD OF PSYCHIATRY AND NEUROLOGY, INC. 06. Congenital disorders 1-3% 07. Cerebrovascular disease 1-3% 08. Neuromuscular diseases 2-4% 09. Cranial nerve palsies 1-3% 10. Spinal cord diseases 1-3% 11. Movement disorders 1-3% 12. Demyelinating diseases 1-3% 13. Neuroinfectious diseases 1-3% 14. Critical care 1-3% 15. Trauma 1-3% 16. Neuro-ophthalmology 1-3% 17. Neuro-otology 1-3% 18. Neurologic complications of systemic diseases 2-4% 19. -

Joubert Syndrome Genereview
Title: Joubert Syndrome GeneReview — Molecular Genetics: Less Common Genetic Causes Authors: Parisi M, Glass I Updated: June 2017 Note: The following information is provided by the authors listed above and has not been reviewed by GeneReviews staff. Joubert Syndrome: Less Common Genetic Causes ARL13B B9D1 B9D2 CEP41 IFT172 KIF7 OFD1 (CXORF5) PDE6D POC1B TCTN1 TCTN3 TMEM138 TMEM231 TMEM237 (ALS2CR4) TTC21B ARL13B Gene structure. ARL13B is a ten-exon gene that encodes a 428-amino acid protein. Pathogenic variants. Two families with a phenotype typical of classic Joubert syndrome had missense and/or nonsense variants in this gene; one of these individuals also had evidence of a retinopathy [Cantagrel et al 2008]. Normal gene product. ARL13B encodes ADP-ribosylation factor-like protein 13B, a member of the ADP-ribosylation factor-like family. Multiple transcript variants result from alternate splicing; two protein isoforms are known. The AR13B protein is a small GTPase in the Ras superfamily that contains both N-terminal and C-terminal guanine nucleotide-binding motifs. It is localized to the cilia and plays a role in cilia formation and maintenance as well as sonic hedgehog signaling. Abnormal gene product. In C elegans, pathogenic variants in the homolog arl13 exhibit defective cilium morphology, localization, and anterograde intraflagellar transport [Cevik et al 2010]. Mice with defects in the murine ortholog have neural tube defects and polydactyly, as well as an embryonic-lethal phenotype [Cantagrel et al 2008, Doherty 2009]. B9D1. See Tables A and B. B9D2. See Tables A and B. CEP41 Gene structure. The gene consists of 11 exons and spans approximately 50 kb. -

Joubert Syndrome and Related Disorders
Brancati et al. Orphanet Journal of Rare Diseases 2010, 5:20 http://www.ojrd.com/content/5/1/20 REVIEW Open Access JoubertReview Syndrome and related disorders Francesco Brancati1,2, Bruno Dallapiccola3 and Enza Maria Valente*1,4 Abstract Joubert syndrome (JS) and related disorders (JSRD) are a group of developmental delay/multiple congenital anomalies syndromes in which the obligatory hallmark is the molar tooth sign (MTS), a complex midbrain-hindbrain malformation visible on brain imaging, first recognized in JS. Estimates of the incidence of JSRD range between 1/80,000 and 1/ 100,000 live births, although these figures may represent an underestimate. The neurological features of JSRD include hypotonia, ataxia, developmental delay, intellectual disability, abnormal eye movements, and neonatal breathing dysregulation. These may be associated with multiorgan involvement, mainly retinal dystrophy, nephronophthisis, hepatic fibrosis and polydactyly, with both inter- and intra-familial variability. JSRD are classified in six phenotypic subgroups: Pure JS; JS with ocular defect; JS with renal defect; JS with oculorenal defects; JS with hepatic defect; JS with orofaciodigital defects. With the exception of rare X-linked recessive cases, JSRD follow autosomal recessive inheritance and are genetically heterogeneous. Ten causative genes have been identified to date, all encoding for proteins of the primary cilium or the centrosome, making JSRD part of an expanding group of diseases called "ciliopathies". Mutational analysis of causative genes is available in few laboratories worldwide on a diagnostic or research basis. Differential diagnosis must consider in particular the other ciliopathies (such as nephronophthisis and Senior-Loken syndrome), distinct cerebellar and brainstem congenital defects and disorders with cerebro-oculo-renal manifestations. -

WES Gene Package Multiple Congenital Anomalie.Xlsx
Whole Exome Sequencing Gene package Multiple congenital anomalie, version 5, 1‐2‐2018 Technical information DNA was enriched using Agilent SureSelect Clinical Research Exome V2 capture and paired‐end sequenced on the Illumina platform (outsourced). The aim is to obtain 8.1 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA‐MEM algorithm (reference: http://bio‐bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A4GALT [Blood group, P1Pk system, P(2) phenotype], 111400 607922 101 100 100 99 [Blood group, P1Pk system, p phenotype], 111400 NOR polyagglutination syndrome, 111400 AAAS Achalasia‐addisonianism‐alacrimia syndrome, 231550 605378 73 100 100 100 AAGAB Keratoderma, palmoplantar, -

Renal Cystic Disorders Infosheet 6-14-19
Next Generation Sequencing Panel for Renal Cystic Disorders Clinical Features: Renal cystic diseases are a genetically heterogeneous group of conditions characterized By isolated renal disease or renal cysts in conjunction with extrarenal features (1). Age of onset of renal cystic disease ranges from neonatal to adult onset. Common features of renal cystic diseases include renal insufficiency and progression to end stage renal disease (ESRD). Identification of the genetic etiology of renal cystic disease can aid in appropriate clinical management of the affected patient. Our Renal Cystic Disorders Panel includes sequence and deletion/duplicaton analysis of all 79 genes listed below. Renal Cystic Disorders Sequencing Panel AHI1 BMPER HNF1B NEK8 TCTN3 WDPCP ANKS6 C5orf42 IFT27 NOTCH2 TFAP2A WDR19 ARL13B CC2D2A IFT140 NPHP1 TMEM107 XPNPEP3 ARL6 CDC73 IFT172 NPHP3 TMEM138 ZNF423 B9D1 CEP104 INPP5E NPHP4 TMEM216 B9D2 CEP120 INVS OFD1 TMEM231 BBIP1 CEP164 IQCB1 PDE6D TMEM237 BBS1 CEP290 JAG1 PKD2 TMEM67 BBS10 CEP41 KIAA0556 PKHD1 TRIM32 BBS12 CEP83 KIAA0586 REN TSC1 BBS2 CRB2 KIF14 RPGRIP1L TSC2 BBS4 CSPP1 KIF7 SALL1 TTC21B BBS5 DCDC2 LZTFL1 SDCCAG8 TTC8 BBS7 GLIS2 MKKS TCTN1 UMOD BBS9 GLIS3 MKS1 TCTN2 VHL Disorder Genes Inheritance Clinical features/molecular genetics Bardet Biedl ARL6 AR Bardet-Biedl syndrome (BBS) is an autosomal syndrome BBS1 recessive multi-systemic ciliopathy characterized By BBS10 retinal dystrophy, oBesity, postaxial polydactyly, BBS12 leaning difficulties, renal involvement and BBS2 genitourinary abnormalities (2). Visual prognosis is BBS4 poor, and the mean age of legal Blindness is 15.5 BBS5 years. Birth weight is typically normal But significant BBS7 weight gain Begins within the first year. Renal BBS9 disease is a major cause of morBidity and mortality. -

Autism Spectrum Disorders—A Genetics Review Judith H
GENETEST REVIEW Genetics in Medicine Autism spectrum disorders—A genetics review Judith H. Miles, MD, PhD TABLE OF CONTENTS Prevalence .........................................................................................................279 Adenylosuccinate lyase deficiency ............................................................285 Clinical features................................................................................................279 Creatine deficiency syndromes..................................................................285 Core autism symptoms...................................................................................279 Smith-Lemli-Opitz syndrome.....................................................................285 Diagnostic criteria and tools..........................................................................280 Other single-gene disorders.......................................................................285 Neurologic and medical symptoms .............................................................281 Developmental syndromes of undetermined etiology..............................286 Genetics of autism...........................................................................................281 Moebius syndrome or sequence...............................................................286 Chromosomal disorders and CNVS..............................................................282 Landau-Kleffner syndrome .........................................................................286 Single-gene -
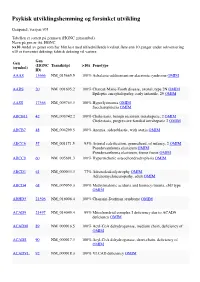
Psykisk Utviklingshemming Og Forsinket Utvikling
Psykisk utviklingshemming og forsinket utvikling Genpanel, versjon v03 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC >x10 Andel av genet som har blitt lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering x10 er forventet dekning; faktisk dekning vil variere. Gen Gen (HGNC Transkript >10x Fenotype (symbol) ID) AAAS 13666 NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AARS 20 NM_001605.2 100% Charcot-Marie-Tooth disease, axonal, type 2N OMIM Epileptic encephalopathy, early infantile, 29 OMIM AASS 17366 NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCB11 42 NM_003742.2 100% Cholestasis, benign recurrent intrahepatic, 2 OMIM Cholestasis, progressive familial intrahepatic 2 OMIM ABCB7 48 NM_004299.5 100% Anemia, sideroblastic, with ataxia OMIM ABCC6 57 NM_001171.5 93% Arterial calcification, generalized, of infancy, 2 OMIM Pseudoxanthoma elasticum OMIM Pseudoxanthoma elasticum, forme fruste OMIM ABCC9 60 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 61 NM_000033.3 77% Adrenoleukodystrophy OMIM Adrenomyeloneuropathy, adult OMIM ABCD4 68 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 21396 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 21497 NM_014049.4 99% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM 89 NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS 90 NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL 92 NM_000018.3 100% VLCAD -

Use of Next Generation Sequencing for Isolated and Syndromic Anophthalmia, Microphthalmia and Coloboma (MAC): a New Approach to Molecular Genetic Diagnosis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archivio della ricerca- Università di Roma La Sapienza Use of Next Generation Sequencing for isolated and syndromic Anophthalmia, Microphthalmia and Coloboma (MAC): a new approach to molecular genetic diagnosis Department of Cellular Biotechnologies and Hematology PhD in Human Biology and Medical Genetics XXX° cycle 2014-2017 Candidate Dr. Iapichino Giuseppe 1235269 Supervisor Correlator Prof. Pizzuti Antonio Prof. Novelli Antonio A/A 2016/2017 1. INTRODUCTION 1.1 Eye embryogenesis 1.2 MAC: Mixed group of diseases 1.2.1 Microphthalmia, Anophthalmia and Coloboma 1.2.2 Syndromic diseases: CHARGE syndrome Cornelia de Lange syndrome Axenfeld-Rieger syndrome Lenz’s Microphtalmia Papillorenal syndrome 1.3 Molecular Diagnosis: Next Generation Sequencing 2. AIM OF THE STUDY 3. MATERIALS AND METHODS 3.1 Patients Recruitment 3.2 Molecular Analysis 3.2.1 Blood collection, DNA extraction and quantification 3.2.2 NGS panel construction: Design Studio 3.2.3 NextSeq-500: features and workflow Libraries preparation: Nextera Rapid Capture Custom Enrichment Cluster generation Sequencing 3.2.4 Sanger Sequencing 3.2.5 Data Analysis 4. RESULTS 5. DISCUSSION 6. CONCLUSION 7. BIBLIOGRAPHY 1. INTRODUCTION 1.1 The eye: embryogenesis Eye development begins around the 4th week of gestation and can be summarized in four main stages [Zagozewski J.L. et al.; 2014]: Optic vesicle formation Induction of crystalline lens Retinal tissue formation Optic fissure closure Each of these events is highly regulated and coordinated by various transcription factors and circulating molecules [Adler R. et al.; 2007]. -

Absence of Mutations in PAX6 Gene in Three Cases of Morning Glory Syndrome Associated with Isolated Growth Hormone Defi Ciency
Absence of Mutations in PAX6 Gene in Three Cases of Morning Glory Syndrome Associated with Isolated Growth Hormone Defi ciency ABSTRACT clinical case report Morning glory syndrome (MGS) is a congenital optic disc dysplasia often as- sociated with craniofacial anomalies, especially basal encephalocele and hy- popituitarism. Clinical signs are varied and often occult. The PAX6 gene is involved in ocular morphogenesis and is expressed in numerous ocular tissues during development especially in the developing central nervous system. The GIL GUERRA-JUNIOR aim of the present study is to evaluate PAX6 in MGS associated with isolated ANGELA MARIA SPINOLA-CASTRO growth hormone defi ciency. Three pre-pubertal males (A, B and C) with MGS and short stature due to growth hormone defi ciency, treated with recombinant ADRIANA A. SIVIERO-MIACHON human growth hormone with limited response, were reported. Two of them ROBERTO GOMES NOGUEIRA had basal encephalocele. Coding and non-coding sequences corresponding of SOFIA HELENA V. L EMOS-MARINI PAX6 different transcripts were analyzed by direct sequencing. Nucleotide vari- ations causing putative aminoacid change were not observed. Patient A pre- LILIA FREIRE RODRIGUES D’SOUZA-LI sented the new IVS2+9G>A transition, whereas patients A and C were PRISCILA CRISTINA DA SILVA heterozygous for known single nucleotide polymorphisms (SNP) within the in- EMERSON SALVADOR S. FRANÇA tron 4. In addition, two SNP heterozygoses were observed for patient C in both intron 9 and 13. Sequencing also revealed several nucleotide variations in pa- FERNANDA CAROLINE SOARDI tient B. Two heterozygoses for known polymorphisms were identifi ed along MARICILDA PALANDI DE MELLO with a novel C>A nucleotide change in intron 4.