Metallurgical Laboratory, with Headquarters at The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VC 1978 1 5.Pdf
Under Contract with the U.S. Department of Energy Vol. No. 1 January 5, 1978 CHRISTMAS BIRD COUNT RESULTS About 9,150 birds of 60 species were spotted in the second annual Fermilab Christmas bird count. The count was conducted Saturday, Dec. 17, by the DuPage Audubon Society. Held in con junction with the national Audubon Society's 78th annual census nationwide, the local tally enlisted 43 volunteer observers, including two Fermilab people. They were: David Carey, Com- puting Department and Hannu Miettinen, Theory . b' d ... Ferm~ ~r -counters were L-R: J. Kumb, Department. R. Johnson, R. Hoger, D. Carey, B. Foster ... Starting at 4 a.m., observers logged 78 hours of bird-counting time. The birders were divided into 11 parties of 4 to 6 persons each; five persons monitored bird feeders during the count. Fermilab was the focal point of the count area: a circle with a radius of seven and one-half miles as far north as Wayne; south to Aurora; east to Winfield; and west to the Fox River Valley. Party-hours comprised 54 on foot, 24 by car and 22 at feeders. Of 425.5 party-miles covered, 367 were by auto and 58.5 on foot. Richard Hoger, staff assistant in the supply division at Argonne National Laboratory, coordinated the count activities. Paul Mooring was the compiler. The Fermilab area was among five Chicago areas where counts were made, Mooring said. Nationally, counters were at work from Dec. 17 to Jan. 2 on one-day counts. Observers were assigned to eight sub-areas in the Laboratory count circle. -
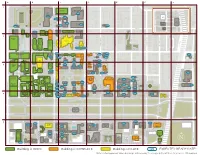
Building Is OPEN Building Is COMPLETE Building Is IN-USE
A B C D E F G E 55TH ST E 55TH ST 1 Campus North Parking Campus North Residential Commons E 52ND ST The Frank and Laura Baker Dining Commons Ratner Stagg Field Athletics Center 5501-25 Ellis Offices - TBD - - TBD - Park Lake S AUG 15 S HARPER AVE Court Cochrane-Woods AUG 15 Art Center Theatre AVE S BLACKSTONE Harper 1452 E. 53rd Court AUG 15 Henry Crown Polsky Ex. Smart Field House - TBD - Alumni Stagg Field Young AUG 15 Museum House - TBD - AUG 15 Building Memorial E 53RD ST E 56TH ST E 56TH ST 1463 E. 53rd Polsky Ex. 5601 S. High Bay West Campus Max Palevsky Commons Max Palevsky Commons Max Palevsky Commons Cottage (2021) Utility Plant AUG 15 Michelson High (West) Energy (Central) (East) 55th, 56th, 57th St Grove Center for Metra Station Physics Physics Child Development TAAC 2 Center - Drexel Accelerator Building Medical Campus Parking B Knapp Knapp Medical Regenstein Library Center for Research William Eckhardt Biomedical Building AVE S KENWOOD Donnelley Research Mansueto Discovery Library Bartlett BSLC Center Commons S Lake Park S MARYLAND AVE S MARYLAND S DREXEL BLVD AVE S DORCHESTER AVE S BLACKSTONE S KIMBARK AVE S UNIVERSITY AVE AVE S WOODLAWN S ELLIS AVE Bixler Park Pritzker Need two weeks to transition School of Biopsychological Medicine Research Building E 57TH ST E 57TH ST - TBD - Rohr Chabad Neubauer Collegium- TBD - Center for Care and Discovery Gordon Center for Kersten Anatomy Center - TBD - Integrative Science Physics Hitchcock Hall Cobb Zoology Hutchinson Quadrangle - TBD - Gate Club Institute of- PoliticsTBD - Snell -
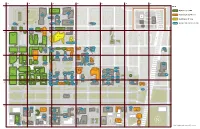
KEY KEY Last Updated: June 15, 2020
Friend Family Health Center Ronald McDonald House A B C D E F G E 55TH ST E 55TH ST KEY 1 Campus North Parking Campus North Residential Commons E 52ND ST The Frank and Laura Baker Dining Commons Building is OPEN Ratner Stagg Field Athletics Center 5501-25 Ellis Offices - TBD - - TBD - Park Lake S Building is COMPLETE AUG 15 S HARPER AVE Court Cochrane-Woods AUG 15 Art Center Theatre AVE S BLACKSTONE Building is IN-USE Harper 1452 E. 53rd Court AUG 15 Henry Crown Polsky Ex. Smart Field House - TBD - Alumni Stagg Field Young AUG 15 Museum House - TBD - DATE EXPECTED READY DATE AUG 15 Building Memorial E 53RD ST E 56TH ST E 56TH ST 1463 E. 53rd Polsky Ex. 5601 S. High Bay West Campus Max Palevsky Commons Max Palevsky Commons Max Palevsky Commons Cottage (2021) Utility Plant AUG 15 Michelson High (West) Energy (Central) (East) 55th, 56th, 57th St Grove Center for Metra Station Physics Physics Child Development TAAC 2 Center - Drexel Accelerator Building Medical Campus Parking B Knapp Knapp Medical Regenstein Library Center for Research William Eckhardt Biomedical Building AVE S KENWOOD Donnelley Research Mansueto Discovery Library Bartlett BSLC Center Commons S Lake Park S KIMBARK AVE S MARYLAND AVE S MARYLAND S DREXEL BLVD AVE S DORCHESTER AVE S BLACKSTONE S UNIVERSITY AVE AVE S WOODLAWN S ELLIS AVE Bixler Park Pritzker Need two weeks to transition School of Biopsychological Medicine Research Building E 57TH ST E 57TH ST - TBD - Rohr Chabad Neubauer CollegiumJUNE 19 Center for Care and Discovery Gordon Center for Kersten Anatomy Center - -

Metallurgical Laboratory (HWMF)
WSRC-TR-94-0615 Unclassified METALLURGICAL LABORATORY HAZARDOUS WASTE MANAGEMENT FACILITY GROUNDWATER MONITORING REPORT (U) FOURTH QUARTER 1994 AND 1994 SUMMARY Publication Date: March 1995 Authorized Derivative Classifier and Reviewing Official: 3-2?-?S UNCLASSIFIED Does Not Contain Unclassified Controlled Nuclear Information Westinghouse Savannah River Company Savannah River Site Aiken, SC 29808 Prepared for the U.S. Department of Energy under Control Contract No. DE-AC09-89SR18035 WSRC-TR-94-0615 Unclassified METALLURGICAL LABORATORY HAZARDOUS WASTE MANAGEMENT FACILITY GROUNDWATER MONITORING REPORT (U) FOURTH QUARTER 1994 AND 1994 SUMMARY Publication Date: March 1995 Authorized Derivative Classifier and Reviewing Official: UNCLASSIFIED Does Not Contain Unclassified Controlled Nuclear Information Westinghouse Savannah River Company Savannah River Site Aiken, SC 29808 DISTRIBUTION OF THIS DOCUMENT IS UNLIMITED'&c Prepared for the U.S. Department of Energy under Control Contract No. DE-AC09-89SR18035 MASTER DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or .assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States * Government or any agency thereof. -

Students on Break, and on a Mission
wishing you a Merry Christmas! Universal preschool gains support The nation’s leading researchers and advocates in the area of early Professionals attending the conference included law and education childhood education gathered this fall at Loyola Law Center for the first professors, national advocacy groups, school board members, preschool national conference on the “Law and Policy of Universal Preschool.” The providers and administrators, state-level board of education reps, and School of Law’s legislators and policy makers represented through their staff. (SOL) ChildLaw “The move to offer universal preschool to all children and their families “The move to offer and Education continues to gain support and has quickly become an important topic universal preschool continues Institute, in nationally,” says Mike Kaufman, professor in the SOL. “The timing for the cooperation conference was perfect, and we were able to offer attendees a positive to gain support...” with the School experience by assembling a list of nationally recognized and well- of Education, respected speakers in both the education and education law fields.” hosted the event, which brought together more than 125 people (90 from outside The conference kicked off with a video welcome from movie director Loyola) to explore the growing movement to ensure early learning access Rob Reiner, a supporter of universal preschool, and was followed by for all children. presentations on the latest research regarding the educational, social, and economic benefits of preschool, as well as the question of preschool access. Closing out the INSIDE conference, universal preschool representatives from Georgia, California, and Illinois shared their experiences in methods to expand preschool access. -

Enrico Fermi: Genius
ANNIVERSARY Enrico Fermi: genius This year marks the centenary of the birth of Enrico Fermi, one of the giants of 20th- • century science, and one of the last physicists to be both an accomplished experimentalist and an influential theorist. Here, Gianni Battimelli of the University of Rome "La Sapienza" traces the life of a genius. Enrico Fermi was born on 29 September 1901 in Rome to a family with no scientific traditions. His passion for natural sciences, and in particular for physics, was stimulated and guided in his school years by an engineer and family friend, Adolph Amidei, who recognized Fermi's exceptional intellectual abilities and suggested admission to Pisa's Scuola Normale Superiore. After finishing high-school studies in Rome, in 1918 Fermi progressed to the prestigious Pisa Institute, after producing for the admission exam an essay on the characteristics of the propagation of sound, the authenticity of which the commissioners initially refused to believe. Studies at Pisa did not pose any particular difficulties for the young Fermi, despite his having to be largely self-taught using mate rial in foreign languages because nothing existed at the time in Fermi's group discovered the Italian on the new physics emerging around relativity and quantum radioactivity induced by theory. In those years in Italy, these new theories were absent from university teaching, and only mathematicians likeTullio Levi-Civita neutrons, instead of the had the knowledge and insight to see their implications. alpha particles used in the Working alone, between 1919 and 1922, Fermi built up a solid competence in relativity, statistical mechanics and the applications Paris experiments. -

Chicago Physics One
CHICAGO PHYSICS ONE 3:25 P.M. December 02, 1942 “All of us... knew that with the advent of the chain reaction, the world would never be the same again.” former UChicago physicist Samuel K. Allison Physics at the University of Chicago has a remarkable history. From Albert Michelson, appointed by our first president William Rainey Harper as the founding head of the physics department and subsequently the first American to win a Nobel Prize in the sciences, through the mid-20th century work led by Enrico Fermi, and onto the extraordinary work being done in the department today, the department has been a constant source of imagination, discovery, and scientific transformation. In both its research and its education at all levels, the Department of Physics instantiates the highest aspirations and values of the University of Chicago. Robert J. Zimmer President, University of Chicago Welcome to the inaugural issue of Chicago Physics! We are proud to present the first issue of Chicago Physics – an annual newsletter that we hope will keep you connected with the Department of Physics at the University of Chicago. This newsletter will introduce to you some of our students, postdocs and staff as well as new members of our faculty. We will share with you good news about successes and recognition and also convey the sad news about the passing of members of our community. You will learn about the ongoing research activities in the Department and about events that took place in the previous year. We hope that you will become involved in the upcoming events that will be announced. -

Argonne National Laboratory Was Founded As a Chemistry, Materials
Physical Sciences and Engineering (PSE) Associate Laboratory Director Requisition 403294 Argonne National Laboratory Lemont, Illinois (Suburb of Chicago) Argonne National Laboratory was founded as a chemistry, materials and nuclear engineering laboratory in 1946, as the successor to the Manhattan Project’s Metallurgical Laboratory. Since then, as part of the Department of Energy (DOE) network of national laboratories, Argonne has built on its original strengths and expanded its mission in response to national needs. Today, Argonne serves America as a leading science and energy laboratory distinguished by the breadth of its research and development (R&D) capabilities combined with a unique portfolio of experimental and computational user facilities. Located just outside Chicago, Argonne has been managed since its founding by The University of Chicago (UChicago), one of the world’s preeminent research universities. Argonne’s workforce of over 3200 includes over 1500 scientists and engineers. The Laboratory operates five world-renowned scientific user facilities, which together support nearly 8,000 researchers annually. Argonne is currently inviting applications for the position of Associate Laboratory Director (ALD) of the Physical Sciences and Engineering (PSE) Directorate, which employs approximately 700 people including scientists, technical and administrative staff, postdocs, fellows, students, visiting scholars and joint appointments and has an annual budget in excess of $200 million. The directorate’s R&D programs have produced a wide range of groundbreaking, internationally recognized discoveries and inventions throughout Argonne’s history. The scope of PSE’s research encompasses materials science, condensed matter physics, chemistry and chemical engineering, and nuclear and particle physics. This work is carried out through five discipline-based operating divisions and is funded primarily by DOE’s Office of Science and Office of Energy Efficiency and Renewable Energy. -

Vol. 6 No. 14 ... Enrico Fermi, Distinguished Physicist, Whose Name Will Head Illinois Research Laboratory ••• ... H. Ande
Vol. 6 No. 14 April11, 1974 The National Accelerator Laboratory will become the Fermi National Accelerator Laboratory at a dedication cere mony to be held at the Laboratory on Saturday, May 11, 1974. The plan to change the name of the Laboratory was announced on April 29, 1969 by Glenn T. Seaborg, then chair man of the U. S. Atomic Energy Commission. It was understood then that the dedication and the changing of the name would take place when construction was complete. May of 1974 will find the Laboratory close to completion and running strongly in all areas. In announcing the AEC's plans, Seaborg said in 1969: ... Enrico Fermi, distinguished "It is particularly fitting that we honor Dr. Fermi in this physicist, whose name will head manner, for in so doing we further acknowledge his many con Illinois research laboratory ••• tributions to the progress of nuclear science, particularly his work on nuclear processes. Enrico Fermi was a physicist of great renown who contributed in a most significant way to the defense and welfare of his adopted land and to the enhancement of its intellectual well-being. His greatest achievement, the first sustained nuclear chain reaction, took place in a small laboratory in Chicago. It seems sin gularly appropriate, therefore, that the Federal Government recognize the memory of a man who was at the forefront of science in his day by naming in his honor a laboratory near Chicago -- a laboratory which will have a major internationa: impact on our understanding of the basic structure of matter." ... H. Anderson, student and long-time Enrico Fermi was born in Rome, Italy, on September 29, colleague of Fermi, on visit to NAL 1901. -

A Doubly Dextrous Physicist Catherine Westfall Lauds a Candid Life of Enrico Fermi, Pioneer of Nuclear Fission
COMMENT BOOKS & ARTS CORBIS VIA GETTY Enrico Fermi in his laboratory in 1931. PHYSICS A doubly dextrous physicist Catherine Westfall lauds a candid life of Enrico Fermi, pioneer of nuclear fission. obel laureate Enrico Fermi con- description of Fermi’s Pontecorvo and Emilio Segré) and put Italy tributed to the discovery of nuclear work so far, as well as on the physics map. fission and was part of the Man- fresh insights into his With this group, Fermi performed Nhattan Project, which built the first atomic personality. (Interest- radio activity-inducing experiments with bombs. Unlike his contemporaries, Fermi ingly, Schwartz’s father, the newfangled neutron, which James was proficient in both theory and experi- physics Nobel laureate Chadwick had discovered in 1932. These ment, knowing everything there was then to Melvin Schwartz, met experiments revealed (among other know about physics. It is therefore surprising Fermi in 1953 and results) that the particles are captured more that political scientist David Schwartz’s new passed up the chance readily when they are slow. The resulting biography is one of just a handful. of working with him.) expertise — and his genius for practical, The Last Man Who Until a few years ago, Fermi featured in Knew Everything: Fermi’s tribulations intuitive problem-solving and painstaking only two full-length accounts. In 1954, the The Life and Times and successes were experimental execution — enabled Fermi year he died, his wife Laura Fermi published of Enrico Fermi, framed in tumult. He to make key advances. He demonstrated the Atoms in the Family, a charming, sometimes Father of the emigrated from Italy first self-sustaining nuclear chain reaction, cheeky account of their marriage and fam- Nuclear Age to the United States in and built the world’s first nuclear reactor ily life. -

The Dupont Company the Forgotten Producers of Plutonium
The DuPont Company The Forgotten Producers of Plutonium Assembled by the “DuPont Story” Committee of the B Reactor Museum Association Ben Johnson, Richard Romanelli, Bert Pierard 2015 Revision 3 – March 2017 FOREWORD Like the world’s tidal waters, the study of our national story sometimes leads us into historical eddies, rich in human interest content, that have been bypassed by the waves of words of the larger accounting of events. Such is the case of the historical accounts of the Manhattan Project which tend to emphasize the triumphs of physicists, while engineering accomplishments, which were particularly important at the Hanford Site, have been brushed over and receive less recognition. The scientific possibility of devising a weapon based on using the energy within the nucleus of the atom was known by physicists in both the United States and Germany before World War II began. After the start of hostilities, these physicists were directed by their respective governments to begin development of atomic bombs. The success of the American program, compared with the German program, was due largely to the extensive involvement in the U.S. Manhattan Project of large and experienced engineering firms whose staff worked with the physicists. The result was the successful production of weapons materials, in an amazingly short time considering the complexity of the program, which helped end World War II. One view which effectively explains these two markedly different historical assessments of accomplishments, at least for Hanford, is noted in the literature with this quote. - "To my way of thinking it was one of the greatest interdisciplinary efforts ever mounted. -

The Enrico Fermi Institute 1
The Enrico Fermi Institute 1 The Enrico Fermi Institute Director • Scott P. Wakely, Physics Professors • Edward Blucher, Physics • John Eric Carlstrom, Astronomy & Astrophysics • Cheng Chin, Physics • Fred Ciesla, Geophysical Sciences • Juan Collar, Physics • Nicolas Dauphas, Geophysical Sciences • Andrew Davis, Geophysical Sciences • Henry J. Frisch, Physics • Lawrence Grossman, Geophysical Sciences • Jeffrey A. Harvey, Physics • Craig Hogan, Astronomy & Astrophysics • Daniel E. Holz, Physics • Wayne Hu, Astronomy & Astrophysics • Alexei Khokhlov, Astronomy & Astrophysics • Young Kee Kim, Physics • Edward W. Kolb, Astronomy & Astrophysics • Arieh Königl, Astronomy & Astrophysics • Andrey Kravtsov, Astronomy & Astrophysics • David Kutasov, Physics • Emil J. Martinec, Physics • Sidney Nagel, Physics • Angela Olinto, Astronomy & Astrophysics • Mark J. Oreglia, Physics • Paolo Privitera, Astronomy & Astrophysics • Robert Rosner, Astronomy & Astrophysics • Savdeep Sethi, Physics • Melvyn Shochet, Physics • Dam Thanh Son, Physics • Michael S. Turner, Astronomy & Astrophysics • Carlos Wagner, Physics • Yau W. Wah, Physics • Scott P. Wakely, Physics • Robert M. Wald, Physics • Liantao Wang, Physics • Paul B. Wiegmann, Physics Part-Time Faculty • Marcela Carena, Professor of Physics (part-time with Fermilab) • Kwang-Je Kim, Professor of Physics (part-time with Argonne) • Michael Pellin, Professor of Geophysical Sciences (part-time with Argonne) • Guy Savard, Professor of Physics (part-time with Argonne) Assistant Professors • Luca Grandi, Physics