Invasion by the Non-Native Earthworm Amynthas Agrestis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

JOURNAL of NEMATOLOGY Molecular And
JOURNAL OF NEMATOLOGY Article | DOI: 10.21307/jofnem-2019-034 e2019-34 | Vol. 51 Molecular and morphological characterization of Paurodontella composticola n. sp. (Nematoda: Hexatylina, Sphaerulariidae) from Iran Mehrab Esmaeili1, Ramin Heydari1,*, Ahmad Kheiri1 and Weimin Ye2 1Department of Plant Protection, Abstract College of Agriculture and natural resources, University of Tehran, A new species of the genus Paurodontella, P. composticola n. sp., Karaj, Iran. collected from Nazar Abad City, Alborz Province, Iran, is described 2Nematode Assay Section, North and illustrated. The new species has a body length of 803–1053 μ m Carolina Department of Agriculture (females n = 10) and 620 and 739 μ m (males n = 2). The cuticle is and Consumer Services, Agronomic weakly annulated with four lateral lines. Cephalic region is annulated Division, Raleigh, NC, 27607, USA. and continuous with body contour. The stylet is 8.0 to 9.0 μ m long *E-mail: [email protected] with asymmetrical knobs. Esophageal basal bulb is present with a small posterior extension projecting into the intestine. Excretory pore This paper was edited by Zafar is situated at the level of esophageal basal bulb region. Post-uterine Ahmad Handoo. sac is 5 to 8 μ m long and uterus is without diverticulum. Tails of both Received for publication November sexes are similar, short and sub-cylindrical. Males have 24 to 25 μ m 20, 2018. long bursa leptoderan and spicules 24 or 25 µm long. A non-branch- ing oviduct is present to form a uterine diverticulum; the new spe- cies is closely related to five known species of the genus, namelyP . -

Three New “Caecate” Earthworm Species from Sulawesi, Indonesia (Oligochaeta, Megascolecidae)
A peer-reviewed open-access journal ZooKeys 805: 1–14 (2018)Three new “caecate” earthworm species from Sulawesi, Indonesia 1 doi: 10.3897/zookeys.805.24834 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Three new “caecate” earthworm species from Sulawesi, Indonesia (Oligochaeta, Megascolecidae) Fahri Fahri1, Rizki Amaliah1, Bambang Suryobroto2, Tri Atmowidi2, Anh D. Nguyen3 1 Department of Biology, Faculty of Mathematics and Natural Sciences, Tadulako University, Jalan Raya Soekarno–Hatta, Tondo, Palu, 94117, Central Sulawesi, Indonesia 2 Department of Biology, Faculty of Ma- thematics and Natural Sciences, Bogor Agricultural University, Dramaga Campus, Bogor 16680, Indonesia 3 Duy Tan University, 254, Nguyen Van Linh, Da Nang city, Vietnam Corresponding author: Fahri Fahri ([email protected]) Academic editor: S. James | Received 6 March 2018 | Accepted 23 October 2018 | Published 11 December 2018 http://zoobank.org/888EA04C-6C61-479D-A134-96125528D032 Citation: Fahri F, Amaliah R, Suryobroto B, Atmowidi T, Nguyen AD (2018) Three new “caecate” earthworm species from Sulawesi, Indonesia (Oligochaeta, Megascolecidae). ZooKeys 805: 1–14. https://doi.org/10.3897/ zookeys.805.24834 Abstract Three new earthworm species are described from Sulawesi, Indonesia. Two belong to the genus Pithemera Sims & Easton, 1972, namely P. suwastikai Fahri, Amaliah & Atmowidi, sp. n. and P. tadulako Fahri, Amaliah & Atmowidi, sp. n. The new species, P. suwastikai sp. n. is distinguished by a medium size (135–165 mm long, 4.5–6.5 mm diameter), four pairs of spermathecal pores in 5/6/7/8/9, 7–12 setae between male pores, no genital markings, holandry, and simple intestinal caeca. Pithemera tadulako sp. -

Taxonomic Assessment of Lumbricidae (Oligochaeta) Earthworm Genera Using DNA Barcodes
European Journal of Soil Biology 48 (2012) 41e47 Contents lists available at SciVerse ScienceDirect European Journal of Soil Biology journal homepage: http://www.elsevier.com/locate/ejsobi Original article Taxonomic assessment of Lumbricidae (Oligochaeta) earthworm genera using DNA barcodes Marcos Pérez-Losada a,*, Rebecca Bloch b, Jesse W. Breinholt c, Markus Pfenninger b, Jorge Domínguez d a CIBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Campus Agrário de Vairão, 4485-661 Vairão, Portugal b Biodiversity and Climate Research Centre, Lab Centre, Biocampus Siesmayerstraße, 60323 Frankfurt am Main, Germany c Department of Biology, Brigham Young University, Provo, UT 84602-5181, USA d Departamento de Ecoloxía e Bioloxía Animal, Universidade de Vigo, E-36310, Spain article info abstract Article history: The family Lumbricidae accounts for the most abundant earthworms in grasslands and agricultural Received 26 May 2011 ecosystems in the Paleartic region. Therefore, they are commonly used as model organisms in studies of Received in revised form soil ecology, biodiversity, biogeography, evolution, conservation, soil contamination and ecotoxicology. 14 October 2011 Despite their biological and economic importance, the taxonomic status and evolutionary relationships Accepted 14 October 2011 of several Lumbricidae genera are still under discussion. Previous studies have shown that cytochrome c Available online 30 October 2011 Handling editor: Stefan Schrader oxidase I (COI) barcode phylogenies are informative at the intrageneric level. Here we generated 19 new COI barcodes for selected Aporrectodea specimens in Pérez-Losada et al. [1] including nine species and 17 Keywords: populations, and combined them with all the COI sequences available in Genbank and Briones et al. -

Symbiosis of the Millipede Parasitic
Nagae et al. BMC Ecol Evo (2021) 21:120 BMC Ecology and Evolution https://doi.org/10.1186/s12862-021-01851-4 RESEARCH ARTICLE Open Access Symbiosis of the millipede parasitic nematodes Rhigonematoidea and Thelastomatoidea with evolutionary diferent origins Seiya Nagae1, Kazuki Sato2, Tsutomu Tanabe3 and Koichi Hasegawa1* Abstract Background: How various host–parasite combinations have been established is an important question in evolution- ary biology. We have previously described two nematode species, Rhigonema naylae and Travassosinema claudiae, which are parasites of the xystodesmid millipede Parafontaria laminata in Aichi Prefecture, Japan. Rhigonema naylae belongs to the superfamily Rhigonematoidea, which exclusively consists of parasites of millipedes. T. claudiae belongs to the superfamily Thelastomatoidea, which includes a wide variety of species that parasitize many invertebrates. These nematodes were isolated together with a high prevalence; however, the phylogenetic, evolutionary, and eco- logical relationships between these two parasitic nematodes and between hosts and parasites are not well known. Results: We collected nine species (11 isolates) of xystodesmid millipedes from seven locations in Japan, and found that all species were co-infected with the parasitic nematodes Rhigonematoidea spp. and Thelastomatoidea spp. We found that the infection prevalence and population densities of Rhigonematoidea spp. were higher than those of Thelastomatoidea spp. However, the population densities of Rhigonematoidea spp. were not negatively afected by co-infection with Thelastomatoidea spp., suggesting that these parasites are not competitive. We also found a positive correlation between the prevalence of parasitic nematodes and host body size. In Rhigonematoidea spp., combina- tions of parasitic nematode groups and host genera seem to be fxed, suggesting the evolution of a more specialized interaction between Rhigonematoidea spp. -

Study on the Biomass and Productivity of Lumbricidae Populations in the Deciduous Ecosystem
Muzeul Olteniei Craiova. Oltenia. Studii úi comunicări. ùtiinĠele Naturii. Tom. 28, No. 2/2012 ISSN 1454-6914 STUDY ON THE BIOMASS AND PRODUCTIVITY OF LUMBRICIDAE POPULATIONS IN THE DECIDUOUS ECOSYSTEM BRÎNZEA GheorghiĠa Abstract. The present study analyses the earthworms in the deciduous ecosystem (Ruginoasa Station) of Cândeúti Platform, in the south-east of Arge܈ County, in terms of biomass and monthly productivity during March-October 2007. Besides numerical dominance, the biomass of Lumbricidae species highlights the role of each species in the activity of matter and energy transfer within an ecosystem. The special dynamics of the two parameters showed different values, given the structure of the vegetation, soil and soil layers, and especially the differences between the individual weights of the species. Octolasion lacteum species was dominant in terms of biomass density and recorded high values at the soil levels analysed, except in the litter. Of the total calculated biomass (27.598 mg d.s./square meter), this species dominates in a ratio of 74.69%. Biological productivity was increased at 0-10 cm, with the highest value for Octolasion lacteum species (4.03 mg d.s./m2), followed by Eisenia lucens species (0.9 mg d.s./m2) and Aporrectodea rosea rosea species (0.641 mg d.s./m2 ). Also, the highest values of the biomass were recorded in spring and autumn months. The species with a lower numerical density recorded low values of the biomass, leading to low biological productivity and vice versa. Keywords: deciduous forest, lumbricidae, biomass, productivity. Rezumat. Studiu privind biomasa úi productivitatea populaĠiilor de Lumbricidae într-un ecosistem de foioase. -
Size Variation and Geographical Distribution of the Luminous Earthworm Pontodrilus Litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan
A peer-reviewed open-access journal ZooKeys 862: 23–43 (2019) Size variation and distribution of Pontodrilus litoralis 23 doi: 10.3897/zookeys.862.35727 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan Teerapong Seesamut1,2,4, Parin Jirapatrasilp2, Ratmanee Chanabun3, Yuichi Oba4, Somsak Panha2 1 Biological Sciences Program, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 2 Ani- mal Systematics Research Unit, Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 3 Program in Animal Science, Faculty of Agriculture Technology, Sakon Nakhon Rajabhat University, Sakon Nakhon 47000, Thailand 4 Department of Environmental Biology, Chubu University, Kasugai 487-8501, Japan Corresponding authors: Somsak Panha ([email protected]), Yuichi Oba ([email protected]) Academic editor: Samuel James | Received 24 April 2019 | Accepted 13 June 2019 | Published 9 July 2019 http://zoobank.org/663444CA-70E2-4533-895A-BF0698461CDF Citation: Seesamut T, Jirapatrasilp P, Chanabun R, Oba Y, Panha S (2019) Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan. ZooKeys 862: 23–42. https://doi.org/10.3897/zookeys.862.35727 Abstract The luminous earthworm Pontodrilus litoralis (Grube, 1855) occurs in a very wide range of subtropical and tropical coastal areas. Morphometrics on size variation (number of segments, body length and diameter) and genetic analysis using the mitochondrial cytochrome c oxidase subunit 1 (COI) gene sequence were conducted on 14 populations of P. -
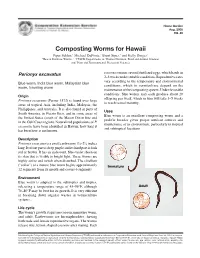
Composting Worms for Hawaii
Home Garden Aug. 2005 HG-46 Composting Worms for Hawaii Piper Selden,1 Michael DuPonte,2 Brent Sipes,3 and Kelly Dinges2 1Hawaii Rainbow Worms, 2, 3CTAHR Departments of 2Human Nutrition, Food and Animal Sciences and 3Plant and Environmental Protection Sciences Perionyx excavatus cocoon contains several fertilized eggs, which hatch in 2–3 weeks under suitable conditions. Reproductive rates Blue worm, India blue worm, Malaysian blue vary according to the temperature and environmental worm, traveling worm conditions, which in vermiculture depend on the maintenance of the composting system. Under favorable Origin conditions, blue worms may each produce about 20 offspring per week, which in turn will take 3–5 weeks Perionyx excavatus (Perrier 1872) is found over large to reach sexual maturity. areas of tropical Asia, including India, Malaysia, the Phillippines, and Australia. It is also found in parts of Uses South America, in Puerto Rico, and in some areas of Blue worm is an excellent composting worm and a the United States (south of the Mason Dixon line and prolific breeder given proper nutrient sources and in the Gulf Coast region). Naturalized populations of P. maintenance of its environment, particularly in tropical excavatus have been identified in Hawaii; how long it and subtropical locations. has been here is not known. Description 1 3 Perionyx excavatus is a small earthworm 1 ⁄4–2 ⁄4 inches long. Its front part is deep purple and its hind part is dark red or brown. It has an iridescent, blue-violet sheen on its skin that is visible in bright light. These worms are highly active and twitch when disturbed. -

The Giant Palouse Earthworm (Driloleirus Americanus)
PETITION TO LIST The Giant Palouse Earthworm (Driloleirus americanus) AS A THREATENED OR ENDANGERED SPECIES UNDER THE ENDANGERED SPECIES ACT June 30, 2009 Friends of the Clearwater Center for Biological Diversity Palouse Audubon Palouse Prairie Foundation Palouse Group of the Sierra Club 1 June 30, 2009 Ken Salazar, Secretary of the Interior Robyn Thorson, Regional Director U.S. Department of the Interior U.S. Fish & Wildlife Service 1849 C Street N.W. Pacific Region Washington, DC 20240 911 NE 11th Ave Portland, Oregon Dear Secretary Salazar, Friends of the Clearwater, Center for Biological Diversity, Palouse Prairie Foundation, Palouse Audubon, Palouse Group of the Sierra Club and Steve Paulson formally petition to list the Giant Palouse Earthworm (Driloleirus americanus) as a threatened or endangered species pursuant to the Endangered Species Act (”ESA”), 16 U.S.C. §1531 et seq. This petition is filed under 5 U.S.C. 553(e) and 50 CFR 424.14 (1990), which grant interested parties the right to petition for issuance of a rule from the Secretary of Interior. Petitioners also request that critical habitat be designated for the Giant Palouse Earthworm concurrent with the listing, pursuant to 50 CFR 424.12, and pursuant to the Administrative Procedures Act (5 U.S.C. 553). The Giant Palouse Earthworm (D. americanus) is found only in the Columbia River Drainages of eastern Washington and Northern Idaho. Only four positive collections of this species have been made within the last 110 years, despite the fact that the earthworm was historically considered “very abundant” (Smith 1897). The four collections include one between Moscow, Idaho and Pullman, Washington, one near Moscow Mountain, Idaho, one at a prairie remnant called Smoot Hill and a fourth specimen near Ellensberg, Washington (Fender and McKey- Fender, 1990, James 2000, Sánchez de León and Johnson-Maynard, 2008). -

Download Download
INSECTA MUNDI A Journal of World Insect Systematics 0238 Snoqualmia, a new polydesmid milliped genus from the northwestern United States, with a description of two new species (Diplopoda, Polydesmida, Polydesmidae) William A. Shear Department of Biology Hampden-Sydney College Hampden-Sydney, VA 23943-0096 U.S.A. Date of Issue: June 15, 2012 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL William A. Shear Snoqualmia, a new polydesmid milliped genus from the northwestern United States, with a description of two new species (Diplopoda, Polydesmida, Polydesmidae) Insecta Mundi 0238: 1-13 Published in 2012 by Center for Systematic Entomology, Inc. P. O. Box 141874 Gainesville, FL 32614-1874 USA http://www.centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non-marine arthropod. Topics considered for publication include systematics, taxonomy, nomencla- ture, checklists, faunal works, and natural history. Insecta Mundi will not consider works in the applied sciences (i.e. medical entomology, pest control research, etc.), and no longer publishes book re- views or editorials. Insecta Mundi publishes original research or discoveries in an inexpensive and timely manner, distributing them free via open access on the internet on the date of publication. Insecta Mundi is referenced or abstracted by several sources including the Zoological Record, CAB Abstracts, etc. Insecta Mundi is published irregularly throughout the year, with completed manu- scripts assigned an individual number. Manuscripts must be peer reviewed prior to submission, after which they are reviewed by the editorial board to ensure quality. One author of each submitted manu- script must be a current member of the Center for Systematic Entomology. -

Ecological Monitoring at Rare, 2020
Ecological Monitoring 2020 rare Charitable Research Reserve Prepared by: Jordan Wrobel Jenna Quinn 1 Acknowledgements Many thanks to Colleges and Institutes Canada (CICan) Career-Launcher Internships, funded by Natural Resource’s Canada’s Green Jobs- Science and Technology Internship Program, and Employment Ontario for providing essential funding for ecological monitoring at rare; without their support, this monitoring program and report would not have been possible. I would also like to thank rare staff for assistance with monitoring and support of intellectual and professional growth. Thank you to Caroline Reisiger and Sarah Cui for their much- appreciated assistance with fieldwork and to Dr. Justin Gaudon for your support with the statistical analyses. To rare’s committed volunteers: Jacqueline Haynes, Miriam Bauman, Emma Wegener, Hilary Irving, Bethany Wakefield, and Logan Mercier; thank you so much for your support with monitoring, these programs would not be as successful without you. I would like to thank all advocates of rare Charitable Research Reserve for helping to support rare’s vision and activities. The rare Charitable Research Reserve acknowledges and is grateful to all of the original stewards of the land in which rare resides, within the Haldimand Tract, spanning six miles on either side of the Grand River from source to mouth. Understanding that this land has been rich in diverse Indigenous presence since time immemorial, there are several Indigenous Nations that we would like to mention. We would like to honor and respect the sovereignty of both First Nations in our area: the Haudenosaunee Peoples of Six Nations of the Grand River and the Anishinaabe Peoples of Mississaugas of the New Credit First Nation. -

A New Species of Illacme Cook & Loomis, 1928
A peer-reviewed open-access journal ZooKeys 626: 1–43A new (2016) species of Illacme Cook and Loomis, 1928 from Sequoia National Park... 1 doi: 10.3897/zookeys.626.9681 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research A new species of Illacme Cook & Loomis, 1928 from Sequoia National Park, California, with a world catalog of the Siphonorhinidae (Diplopoda, Siphonophorida) Paul E. Marek1, Jean K. Krejca2, William A. Shear3 1 Virginia Polytechnic Institute and State University, Department of Entomology, Price Hall, Blacksburg, Virginia, USA 2 Zara Environmental LLC, 1707 W FM 1626, Manchaca, Texas, USA 3 Hampden-Sydney College, Department of Biology, Gilmer Hall, Hampden-Sydney, Virginia, USA Corresponding author: Paul E. Marek ([email protected]) Academic editor: R. Mesibov | Received 25 July 2016 | Accepted 19 September 2016 | Published 20 October 2016 http://zoobank.org/36E16503-BC2B-4D92-982E-FC2088094C93 Citation: Marek PE, Krejca JK, Shear WA (2016) A new species of Illacme Cook & Loomis, 1928 from Sequoia National Park, California, with a world catalog of the Siphonorhinidae (Diplopoda, Siphonophorida). ZooKeys 626: 1–43. doi: 10.3897/zookeys.626.9681 Abstract Members of the family Siphonorhinidae Cook, 1895 are thread-like eyeless millipedes that possess an astounding number of legs, including one individual with 750. Due to their cryptic lifestyle, rarity in natural history collections, and sporadic study over the last century, the family has an unclear phylogenetic placement, and intrafamilial relationships remain unknown. Here we report the discovery of a second spe- cies of Illacme, a millipede genus notable for possessing the greatest number of legs of any known animal on the planet. -

Amynthas) in the Northeast United States
Invertebrate Biology x(x): 1–14. © 2016 The Authors. Invertebrate Biology published by Wiley Periodicals, Inc. on behalf of American Microscopical Society. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made. DOI: 10.1111/ivb.12145 Phylogeographic analysis of invasive Asian earthworms (Amynthas) in the northeast United States Nancy Schult, Kelly Pittenger, Sam Davalos, and Damhnait McHugha Department of Biology, Colgate University, Hamilton, New York 13346, USA Abstract. Phylogeographic studies are useful in reconstructing the history of species inva- sions, and in some instances can elucidate cryptic diversity of invading taxa. This can help in predicting or managing the spread of invasive species. Among terrestrial invasive species in North America, earthworms can have profound ecological effects. We are familiar with the centuries-old invasions of European earthworms (Lumbricidae) and their impacts on nutrient cycling in soils. More recent invasions by Asian earthworms of the family Megascolecidae are less fully understood. We used data for two mitochondrial gene frag- ments, cytochrome oxidase I (COI) and 16S rRNA, to examine the relationships among populations of Asian earthworms in the megascolecid genus Amynthas in the northeast Uni- ted States. Recent reports have indicated that one species in particular, Amynthas agrestis, is having detrimental effects in mixed forest ecosystems, and we were interested in under- standing the invasion history for this species. We were surprised to discover three divergent mitochondrial lineages of Amynthas occurring sympatrically in upstate New York.