Download Product Insert
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recommended Methods for the Identification and Analysis of Synthetic Cathinones in Seized Materialsd
Recommended methods for the Identification and Analysis of Synthetic Cathinones in Seized Materials (Revised and updated) MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Photo credits:UNODC Photo Library; UNODC/Ioulia Kondratovitch; Alessandro Scotti. Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Synthetic Cathinones in Seized Materials (Revised and updated) MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2020 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/49/REV.1 Original language: English © United Nations, March 2020. All rights reserved, worldwide. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. -

Free PDF Download
European Review for Medical and Pharmacological Sciences 2019; 23: 3-15 Use of cognitive enhancers: methylphenidate and analogs J. CARLIER1, R. GIORGETTI2, M.R. VARÌ3, F. PIRANI2, G. RICCI4, F.P. BUSARDÒ2 1Unit of Forensic Toxicology, Sapienza University of Rome, Rome, Italy 2Section of Legal Medicine, Universita Politecnica delle Marche, Ancona, Italy 3National Centre on Addiction and Doping, Istituto Superiore di Sanità, Rome, Italy 4School of Law, University of Camerino, Camerino, Italy Abstract. – OBJECTIVE: In the last decades, phenidate analogs should be undertaken to re- several cognitive-enhancing drugs have been duce the uprising threat, and education efforts sold onto the drug market. Methylphenidate and should be made among high-risk populations. analogs represent a sub-class of these new psy- choactive substances (NPS). We aimed to re- Key Words: view the use and misuse of methylphenidate and Cognitive enhancers, Methylphenidate, Ritalin, Eth- analogs, and the risk associated. Moreover, we ylphenidate, Methylphenidate analogs, New psycho- exhaustively reviewed the scientific data on the active substances. most recent methylphenidate analogs (methyl- phenidate and ethylphenidate excluded). MATERIALS AND METHODS: Literature Introduction search was performed on methylphenidate and analogs, using specialized search engines ac- cessing scientific databases. Additional reports Consumption of various pharmaceutical drugs were retrieved from international agencies, in- by healthy individuals in an attempt to improve stitutional websites, and drug user forums. cognitive faculties is on the rise, whether for aca- RESULTS: Methylphenidate/Ritalin has been demic or recreational purposes1. These substances used for decades to treat attention deficit disor- are stimulants that preferentially target the cate- ders and narcolepsy. More recently, it has been used as a cognitive enhancer and a recreation- cholamines of the prefrontal cortex of the brain to al drug. -

CAS Number Index
2334 CAS Number Index CAS # Page Name CAS # Page Name CAS # Page Name 50-00-0 905 Formaldehyde 56-81-5 967 Glycerol 61-90-5 1135 Leucine 50-02-2 596 Dexamethasone 56-85-9 963 Glutamine 62-44-2 1640 Phenacetin 50-06-6 1654 Phenobarbital 57-00-1 514 Creatine 62-46-4 1166 α-Lipoic acid 50-11-3 1288 Metharbital 57-22-7 2229 Vincristine 62-53-3 131 Aniline 50-12-4 1245 Mephenytoin 57-24-9 1950 Strychnine 62-73-7 626 Dichlorvos 50-23-7 1017 Hydrocortisone 57-27-2 1428 Morphine 63-05-8 127 Androstenedione 50-24-8 1739 Prednisolone 57-41-0 1672 Phenytoin 63-25-2 335 Carbaryl 50-29-3 569 DDT 57-42-1 1239 Meperidine 63-75-2 142 Arecoline 50-33-9 1666 Phenylbutazone 57-43-2 108 Amobarbital 64-04-0 1648 Phenethylamine 50-34-0 1770 Propantheline bromide 57-44-3 191 Barbital 64-13-1 1308 p-Methoxyamphetamine 50-35-1 2054 Thalidomide 57-47-6 1683 Physostigmine 64-17-5 784 Ethanol 50-36-2 497 Cocaine 57-53-4 1249 Meprobamate 64-18-6 909 Formic acid 50-37-3 1197 Lysergic acid diethylamide 57-55-6 1782 Propylene glycol 64-77-7 2104 Tolbutamide 50-44-2 1253 6-Mercaptopurine 57-66-9 1751 Probenecid 64-86-8 506 Colchicine 50-47-5 589 Desipramine 57-74-9 398 Chlordane 65-23-6 1802 Pyridoxine 50-48-6 103 Amitriptyline 57-92-1 1947 Streptomycin 65-29-2 931 Gallamine 50-49-7 1053 Imipramine 57-94-3 2179 Tubocurarine chloride 65-45-2 1888 Salicylamide 50-52-2 2071 Thioridazine 57-96-5 1966 Sulfinpyrazone 65-49-6 98 p-Aminosalicylic acid 50-53-3 426 Chlorpromazine 58-00-4 138 Apomorphine 66-76-2 632 Dicumarol 50-55-5 1841 Reserpine 58-05-9 1136 Leucovorin 66-79-5 -

Article 22 Regulation for Restriction of Synthetic Drugs
ARTICLE 22 REGULATION FOR RESTRICTION OF SYNTHETIC DRUGS SECTION 22.1 AUTHORITY This regulation is promulgated under the authority granted to the Needham Board of Health under Massachusetts General Laws Chapter 111, Section 31 which states that “boards of health may make reasonable health regulations”. SECTION 22.2 PURPOSE The Needham Board of Health has found that synthetic marijuana, consisting of plant or other material treated with various chemicals or other synthetic substances not approved for human consumption, may be marketed and sold as herbal incense in the greater Boston area, although they are being used in the same manner and for the same purposes as scheduled drugs. In addition, the use of these products has become particularly popular among teens and young adults. Based on information and reports from hospitals, emergency room doctors, and police agencies, individuals who use these products experience dangerous side effects including convulsions, hallucinations, and dangerously elevated heart rates. This is evidence that synthetic marijuana products are harmful if inhaled or consumed, and present a significant public health danger. These synthetic compounds and others have a high potential for abuse and lack of any accepted medical use, these dangerous products, while not approved for human consumption, are marketed and sold in a form that allows for such consumption, putting at risk the individuals who come into contact with them. Therefore, the Needham Board of Health adopts this regulation for the purpose and with the intent to protect the public health and safety of the Town of Needham and its residents from the threat posed by the availability and use of synthetic marijuana, synthetic stimulants, synthetic hallucinogens, and other dangerous products by prohibiting persons from trafficking in, possessing, and using them within the town. -

AGENDA Friday, September 9, 2016 7:00 A.M
Needham Board of Health AGENDA Friday, September 9, 2016 7:00 a.m. – 9:00 a.m. Charles River Room – Public Services Administration Building 500 Dedham Avenue, Needham MA 02492 • 7:00 to 7:05 - Welcome & Review of Minutes (July 29 & August 29) • 7:05 to 7:30 - Director and Staff Reports (July & August) • 7:30 to 7:45 - Discussion about Proposed Plastic Bag Ban Christopher Thomas, Needham Resident • 7:45 to 7:50 - Off-Street Drainage Bond Discussion & Vote • 7:50 to 8:00 - Update on Wingate Pool Variance Application * * * * * * * * * * * * * Board of Health Public Hearing • 8:00 to 8:40 - Hearing for Proposed New or Amended BOH Regulations o Body Art o Synthetic Marijuana o Drug Paraphernalia • 8:40 to 8:50 - Board Discussion of Policy Positions • Other Items (Healthy Aging, Water Quality) • Next Meeting Scheduled for Friday October 14, 2016 • Adjournment (Please note that all times are approximate) 1471 Highland Avenue, Needham, MA 02492 781-455-7500 ext 511 (tel); 781-455-0892 (fax) E-mail: [email protected] Web: www.needhamma.gov/health NEEDHAM BOARD OF HEALTH July 29, 2016 MEETING MINUTES PRESENT: Edward V. Cosgrove, PhD, Chair, Jane Fogg, Vice-Chair, M.D., and Stephen Epstein, M.D STAFF: Timothy McDonald, Director, Donna Carmichael, Catherine Delano, Maryanne Dinell, Tara Gurge GUEST: Kevin Mulkern, Aquaknot Pools, Inc., Keith Mulkern, Aquaknot Pools, Inc., David Friedman, Wingate, Paul Humphreys, Michael Tomasello, Callahan, Inc. CONVENE: 7:00 a.m. – Public Services Administration Building (PSAB), 500 Dedham Avenue, Needham MA 02492 DISCUSSION: Call To Order – 7:06 a.m. – Dr. Cosgrove, Chairman APPROVE MINUTES: Upon motion duly made and seconded, the minutes of the BOH meeting of June 17, 2016 were approved as submitted. -

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved. -

Model Scheduling New/Novel Psychoactive Substances Act (Third Edition)
Model Scheduling New/Novel Psychoactive Substances Act (Third Edition) July 1, 2019. This project was supported by Grant No. G1799ONDCP03A, awarded by the Office of National Drug Control Policy. Points of view or opinions in this document are those of the author and do not necessarily represent the official position or policies of the Office of National Drug Control Policy or the United States Government. © 2019 NATIONAL ALLIANCE FOR MODEL STATE DRUG LAWS. This document may be reproduced for non-commercial purposes with full attribution to the National Alliance for Model State Drug Laws. Please contact NAMSDL at [email protected] or (703) 229-4954 with any questions about the Model Language. This document is intended for educational purposes only and does not constitute legal advice or opinion. Headquarters Office: NATIONAL ALLIANCE FOR MODEL STATE DRUG 1 LAWS, 1335 North Front Street, First Floor, Harrisburg, PA, 17102-2629. Model Scheduling New/Novel Psychoactive Substances Act (Third Edition)1 Table of Contents 3 Policy Statement and Background 5 Highlights 6 Section I – Short Title 6 Section II – Purpose 6 Section III – Synthetic Cannabinoids 13 Section IV – Substituted Cathinones 19 Section V – Substituted Phenethylamines 23 Section VI – N-benzyl Phenethylamine Compounds 25 Section VII – Substituted Tryptamines 28 Section VIII – Substituted Phenylcyclohexylamines 30 Section IX – Fentanyl Derivatives 39 Section X – Unclassified NPS 43 Appendix 1 Second edition published in September 2018; first edition published in 2014. Content in red bold first added in third edition. © 2019 NATIONAL ALLIANCE FOR MODEL STATE DRUG LAWS. This document may be reproduced for non-commercial purposes with full attribution to the National Alliance for Model State Drug Laws. -

Appendix-2Final.Pdf 663.7 KB
North West ‘Through the Gate Substance Misuse Services’ Drug Testing Project Appendix 2 – Analytical methodologies Overview Urine samples were analysed using three methodologies. The first methodology (General Screen) was designed to cover a wide range of analytes (drugs) and was used for all analytes other than the synthetic cannabinoid receptor agonists (SCRAs). The analyte coverage included a broad range of commonly prescribed drugs including over the counter medications, commonly misused drugs and metabolites of many of the compounds too. This approach provided a very powerful drug screening tool to investigate drug use/misuse before and whilst in prison. The second methodology (SCRA Screen) was specifically designed for SCRAs and targets only those compounds. This was a very sensitive methodology with a method capability of sub 100pg/ml for over 600 SCRAs and their metabolites. Both methodologies utilised full scan high resolution accurate mass LCMS technologies that allowed a non-targeted approach to data acquisition and the ability to retrospectively review data. The non-targeted approach to data acquisition effectively means that the analyte coverage of the data acquisition was unlimited. The only limiting factors were related to the chemical nature of the analyte being looked for. The analyte must extract in the sample preparation process; it must chromatograph and it must ionise under the conditions used by the mass spectrometer interface. The final limiting factor was presence in the data processing database. The subsequent study of negative MDT samples across the North West and London and the South East used a GCMS methodology for anabolic steroids in addition to the General and SCRA screens. -

FEWS Annual Report 15/16
Report on the Home Office Forensic Early Warning System (FEWS) – 2015/16 A system to identify New Psychoactive Substances (NPS) in the UK November 2018 Contents Executive summary 3 Background to FEWS 5 Analysis of results and findings 6 Action taken by Government to tackle NPS 13 Looking forward 15 2 1. Executive summary The Home Office Forensic Early Warning System (FEWS) was set up in 2011 in response to the emergence of new psychoactive substances (NPS). NPS are mainly synthetic drugs manufactured in a laboratory or factory (chiefly based overseas) and are used in the UK, Europe and the rest of the world. Over 500 NPS are controlled under the Misuse of Drugs Act 1971 (MDA) but there are many more that are not controlled. During 2015/16 FEWS: • obtained and analysed 2,386 samples from six FEWS collection plans including attending one UK music festival with an on-site laboratory. • provided support for the Advisory Council on the Misuse of Drugs’ (ACMD) ongoing monitoring of NPS; and • supported decisions relating to the Psychoactive Substances Act 2016. FEWS collected samples from the internet, head shops1, a music festival, prisons and through police forces, to identify NPS which are present in the UK or being offered for sale in the UK. The key aim of the collection plans was to collect information on NPS that are being seen in a cross-section of different environments. Key findings During 2015/16, out of 2,386 samples seen under FEWS, 1,429 samples contained at least one NPS and of these, 489 samples contained an NPS that was controlled under the MDA at the time of seizure2, while 940 samples contained an NPS which was not controlled. -
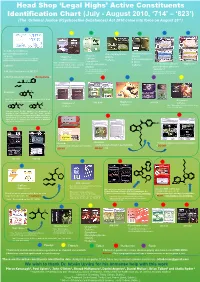
Identification Chart (July
Head Shop ‘Legal Highs’ Active Constituents Identification Chart ((yJuly - August 2010, ‘714’ – ‘823’) (The Criminal Justice (Psychoactive Substances) Act 2010 came into force on August 23rd ) •3', 4'-MthlMethylene dioxy-α- pyrrolidinobutiophenone •Dimethylamylamine •Dimethylamylamine •Glaucine* (MDPBP) •Glaucine* (DMAA) (DMAA) (identified using a characterized sample of MDPBP •Caffeine •Caffeine (trace) •Caffeine •2-Phenylethylamine extracted from Vanilla Sky as a reference standard) •Synephrine Note: GCMS analysis also revealed (2-PEA) •Dimethylamylamine •3',4'-Methylenedioxy-α- •Naphyrone† •Caffeine several unidentified peaks. Could be •Caffeine compounds related to glaucine? (DMAA) pyrrolidinobutiophenone ¤ Note: GCMS analysis also revealed (MDPBP) † •4-Methylethcathinone (4-MEC) an unidentified peak. Could be a Note: This product contained MDPV compound related to glaucine? and lignocaine before the ‘511’ ban. •4-Methyl-N-benzylcathinone (‘Benzedrone’)† O H N O H •Pentylone* O N O Note: We also considered the isomers below which would be expected to have similar mass spectra. † •Benzocaine O O † •Naphyrone •AM-694 * H •Naphyrone •AM-694 * •Caffeine O N O N •Caffeine •Caffeine Note: This product contained mephedrone O O before the ‘511’ ban. However, based on first principles, the preparation of derivatives and comparison with the mass spectra analogous molecules, the mass spectral data most closely fits pentylone. In this case the mass spectrum displays a significant m/z 44 ion which may arise by loss of propylene O from m/z 86 iminium ion. OMe N •Oleamide •Oleamide •3-(4-Methoxybenzoyl)-1-pentylindole •3-(4-Methoxybenzoyl)-1-pentylindole •3-(4-Methoxybenzoyl)-1-pentylindole (‘DD-001’) † (‘DD-001’) † (‘DD-001’) † ¤ •AM-694* •AM-694* O O N N N N •Dimethocaine* O •Caffeine Note: Also contains desethyl dimethocaine. -

Alcohol and Drug Abuse Subchapter 9
Chapter 8 – Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority This rule is established under the authority of 18 V.S.A. §§ 4201 and 4202 which authorizes the Vermont Board of Health to designate regulated drugs for the protection of public health and safety. 2.0 Purpose This rule designates drugs and other chemical substances that are illegal or judged to be potentially fatal or harmful for human consumption unless prescribed and dispensed by a professional licensed to prescribe or dispense them and used in accordance with the prescription. The rule restricts the possession of certain drugs above a specified quantity. The rule also establishes benchmark unlawful dosages for certain drugs to provide a baseline for use by prosecutors to seek enhanced penalties for possession of higher quantities of the drug in accordance with multipliers found at 18 V.S.A. § 4234. 3.0 Definitions 3.1 “Analog” means one of a group of chemical components similar in structure but different with respect to elemental composition. It can differ in one or more atoms, functional groups or substructures, which are replaced with other atoms, groups or substructures. 3.2 “Benchmark Unlawful Dosage” means the quantity of a drug commonly consumed over a twenty-four-hour period for any therapeutic purpose, as established by the manufacturer of the drug. Benchmark Unlawful dosage is not a medical or pharmacologic concept with any implication for medical practice. Instead, it is a legal concept established only for the purpose of calculating penalties for improper sale, possession, or dispensing of drugs pursuant to 18 V.S.A. -

NFLIS-Drug Selected Substance List
2017-2020 NFLIS-Drug Substance List (Sorted by Date) Date Added NFLIS Substance Name Synonyms Chemical Name Structure InChI Formula to NFLIS- Drug InChI=1S/C16H20BrN/ c17-14-1-3-15(4-2-14)18-16-12-6-10-5-11 Bromantane ladasten N-(4-bromophenyl)adamantan-2-amine C16H20BrN 12/7/20 (8-12)9-13(16)7-10/h1-4,10-13,16,18H, 5-9H2 InChI=1S/C21H29FN2O3/ c1-4-27-21(26)19(15(2)3)23-20(25)17-14- ethyl 2-(1-(5-fluoropentyl)-1H-indole-3-carboxamido)-3- 5F-EMB-PICA EMB-2201; 5-fluoro-EMB-PICA 24(13-9-5-8-12-22)18-11-7-6-10-16(17)18 C21H29FN2O3 11/12/20 methylbutanoate /h6-7,10-11,14-15,19H, 4-5,8-9,12-13H2,1-3H3,(H,23,25) InChI=1S/C20H27FN2O3/ c1-20(2,3)17(19(25)26-4)22-18(24)15-13- methyl 2-(1-(4-fluorobutyl)-1H-indole-3- 4F-MDMB-BUTICA 4-fluoro-MDMB-BUTICA; 4F-MDMB-BICA 23(12-8-7-11-21)16-10-6-5-9-14(15)16/ C20H27FN2O3 10/23/20 carboxamido)-3,3-dimethylbutanoate h5-6,9-10,13,17H,7-8,11-12H2,1-4H3,(H, 22,24) InChI=1S/C10H14BrNO2/ 4-methoxy-6-[(1E)-2-phenylethenyl]-5,6-dihydro-2H- 2Br-4,5-Dimethoxyphenethylamine 2-bromo-4,5-dimethoxyphenethylamine c1-13-9-5-7(3-4-12)8(11)6-10(9)14-2/ C10H14BrNO2 10/2/20 pyran-2-one h5-6H,3-4,12H2,1-2H3 InChI=1S/C16H22FNO/ 4-fluoro-3-methyl-alpha-PVP; 4F-3-methyl-alpha- c1-3-6-15(18-9-4-5-10-18)16(19)13-7-8-1 4F-3-Methyl-alpha-PVP 4-fluoro-3-methyl-alpha-pyrrolidinopentiophenone C16H22FNO 10/2/20 pyrrolidinovalerophenone 4(17)12(2)11-13/h7-8,11,15H, 3-6,9-10H2,1-2H3 InChI=1S/C21H26N4O3/ N,N-diethyl-2-[2-(4-methoxybenzyl)-5-nitro-1H- c1-4-23(5-2)12-13-24-20-11-8-17(25(26)2 Metonitazene C21H26N4O3 9/15/20 benzimidazol-1-yl]ethanamine