Phylogeny of the Family Sialidae (Insecta: Megaloptera) Inferred from Morphological Data, with Implications for Generic Classification and Historical Biogeography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aquatic Insects Are Dramatically Underrepresented in Genomic Research
insects Communication Aquatic Insects Are Dramatically Underrepresented in Genomic Research Scott Hotaling 1,* , Joanna L. Kelley 1 and Paul B. Frandsen 2,3,* 1 School of Biological Sciences, Washington State University, Pullman, WA 99164, USA; [email protected] 2 Department of Plant and Wildlife Sciences, Brigham Young University, Provo, UT 84062, USA 3 Data Science Lab, Smithsonian Institution, Washington, DC 20002, USA * Correspondence: [email protected] (S.H.); [email protected] (P.B.F.); Tel.: +1-(828)-507-9950 (S.H.); +1-(801)-422-2283 (P.B.F.) Received: 20 August 2020; Accepted: 3 September 2020; Published: 5 September 2020 Simple Summary: The genome is the basic evolutionary unit underpinning life on Earth. Knowing its sequence, including the many thousands of genes coding for proteins in an organism, empowers scientific discovery for both the focal organism and related species. Aquatic insects represent 10% of all insect diversity, can be found on every continent except Antarctica, and are key components of freshwater ecosystems. However, aquatic insect genome biology lags dramatically behind that of terrestrial insects. If genomic effort was spread evenly, one aquatic insect genome would be sequenced for every ~9 terrestrial insect genomes. Instead, ~24 terrestrial insect genomes have been sequenced for every aquatic insect genome. A lack of aquatic genomes is limiting research progress in the field at both fundamental and applied scales. We argue that the limited availability of aquatic insect genomes is not due to practical limitations—small body sizes or overly complex genomes—but instead reflects a lack of research interest. We call for targeted efforts to expand the availability of aquatic insect genomic resources to empower future research. -

Viewed Erature to Ensure the Most Up-To-Date Treatment with Caution, P~Rticularlyamong Older Literature
PROCEEDINGS OF THE CALIFORNIA ACADEMY OF SCIENCES Vol. 50, No. 3, pp. 39-114. December 9, 1997 SPECIES CATALOG OF THE NEUROPTERA, MEGALOPTERA, AND RAPHIDIOPTERA OF AMERlCA NORTH OF MEXICO Norman D. Penny Department ofE~ztorizolog)~,Caldornla Acndony oJ'Sc~erzces, San Fmnc~sco,CA 941 18 Phillip A. Adams Ccllg'rnia State Utzivet-sity, F~lllet-ton,CA 92634 and Lionel A. Stange Florida Depat>tnzen/oj'Agt.~czi/trrre, Gr~~nesv~/le, FL 32602 Thc 399 currently recognized valid species of the orders Neuroptera, Megaloptera, and Raphidioptera that are known to occur in America north of Mexico are listed and full synonymies given. Geographical distributions are listed by states and province\. Complete bibliographic references are given for all namcs and nomenclatural acts. Included are two new Junior homonyms indicated, seven new taxonomic cornbinations, two new changes of rank, fourteen new synonymies, three new lectotype de\ignations, and onc new name. Received March 20,1996. Accepted June 3, 1997. The recent publication of Nomina Insecta been consulted whenever possible, as well as Nearctica, A Check List of the Insects of North Zoological Record, and appropriate mono- America (Poole 1996) has given us a listing of graphic revisions publishedup to 1 January 1997. North American Neuropterida (Neuroptera + A number of taxonomic changes are incorpo- Megaloptera + Raphidioptera) species for the rated into this catalog: there are two new Junior first tlme in more than a century. However, for homonyms indicated, seven new taxonomic anyone trying to identify these species, the litera- combinations, two new changes of rank. fourteen ture is scattered and obscure. -

Insecta, Neuropterida, Megaloptera, Sialidae)
Graellsia, 70(2): e009 julio-diciembre 2014 ISSN-L: 0367-5041 http://dx.doi.org/10.3989/graellsia.2014.v70.111 LOS MEGALÓPTEROS DE LA PENÍNSULA IBÉRICA (INSECTA, NEUROPTERIDA, MEGALOPTERA, SIALIDAE) Víctor J. Monserrat Departamento de Zoología y Antropología Física, Facultad de Biología, Universidad Complutense, E-28040 Madrid, España. E-mail: [email protected] RESUMEN Se actualiza toda la información bibliográfica relativa a la Península Ibérica y relacionada con las tres especies de megalópteros presentes en su fauna (Insecta, Neuropterida, Megaloptera: Sialidae). Partiendo de los datos generales conocidos sobre estas especies, y en base a esta información ibérica, se aporta una clave de identifi- cación de imagos y larvas de estas especies, y se anotan y se recopilan los datos conocidos sobre su morfología, su biología, sus estadios larvarios y su distribución geográfica, fenológica y altitudinal en la zona estudiada. Palabras clave: Península Ibérica; Faunística; Biología; Neuropterida; Megaloptera; Sialidae; Sialis; “monjas”. ABSTRACT The alder-flies of the Iberian Peninsula (Insecta, Neuropterida, Megaloptera, Sialidae) All existing Iberian bibliographical information related to the three alder-flies species known in the Iberian Peninsula’s fauna (Insecta, Neuropterida, Megaloptera: Sialidae) is brought up to date. On the basis of general knowledge about these species, and taking into account the known Iberian data, a key for imagoes and larvae is included and what is known about their morphology, biology, larval stages and geographical, phenological and altitudinal distribution in the area studied is reviewed. Keywords: Iberian Peninsula; Faunistical; Biology; Neuropterida; Megaloptera; Sialidae; Sialis; “alder-flies”. Recibido/Received: 14/03/2014; Aceptado/Accepted: 02/09/2014; Publicado en línea/Published online: 26/11/2014 Como citar este artículo/Citation: Monserrat, V. -

Lessons from Genome Skimming of Arthropod-Preserving Ethanol Benjamin Linard, P
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archive Ouverte en Sciences de l'Information et de la Communication Lessons from genome skimming of arthropod-preserving ethanol Benjamin Linard, P. Arribas, C. Andújar, A. Crampton-Platt, A. P. Vogler To cite this version: Benjamin Linard, P. Arribas, C. Andújar, A. Crampton-Platt, A. P. Vogler. Lessons from genome skimming of arthropod-preserving ethanol. Molecular Ecology Resources, Wiley/Blackwell, 2016, 16 (6), pp.1365-1377. 10.1111/1755-0998.12539. hal-01636888 HAL Id: hal-01636888 https://hal.archives-ouvertes.fr/hal-01636888 Submitted on 17 Jan 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Lessons from genome skimming of arthropod-preserving 2 ethanol 3 Linard B.*1,4, Arribas P.*1,2,5, Andújar C.1,2, Crampton-Platt A.1,3, Vogler A.P. 1,2 4 5 1 Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 6 5BD, UK, 7 2 Department of Life Sciences, Imperial College London, Silwood Park Campus, Ascot 8 SL5 7PY, UK, 9 3 Department -

Winter Behavior and Ecology of Eastern Bluebirds (<I
Eastern Kentucky University Encompass Online Theses and Dissertations Student Scholarship January 2013 Winter Behavior and Ecology of Eastern Bluebirds (Sialia sialis): Home Ranges, Habitat Use, and Effect of Weather on Foraging Behavior Todd J. Weinkam Eastern Kentucky University Follow this and additional works at: https://encompass.eku.edu/etd Part of the Ecology and Evolutionary Biology Commons, and the Ornithology Commons Recommended Citation Weinkam, Todd J., "Winter Behavior and Ecology of Eastern Bluebirds (Sialia sialis): Home Ranges, Habitat Use, and Effect of Weather on Foraging Behavior" (2013). Online Theses and Dissertations. 144. https://encompass.eku.edu/etd/144 This Open Access Thesis is brought to you for free and open access by the Student Scholarship at Encompass. It has been accepted for inclusion in Online Theses and Dissertations by an authorized administrator of Encompass. For more information, please contact [email protected]. WINTER BEHAVIOR AND ECOLOGY OF EASTERN BLUEBIRDS (Sialia sialis): HOME RANGES, HABITAT USE, AND EFFECT OF WEATHER ON FORAGING BEHAVIOR By: Todd J. Weinkam Bachelor of Science Ohio University Athens, Ohio 2008 Submitted to the Faculty of the Graduate School of Eastern Kentucky University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE December, 2013 Copyright © 2013 by Todd J. Weinkam All rights reserved ii DEDICATION This thesis is dedicated to my parents, Jerry and Marcia, without the support of whom my aspirations, educational and otherwise, would have been impossible. To Cara, for her continuing encouragement every day. And To you, the reader: May you learn something interesting. iii ACKNOWLEDGMENTS I am grateful for the extensive help and guidance I’ve received throughout the process of this thesis. -

Life History and Production Studies of Sialis
AN ABSTRACT OF THE THESIS OF KHAJA MOHAMMED AZAM for the DOCTOR OF PHILOSOPHY (Name) (Degree) in ENTOMOLOGY presented on June 17, 1968 (Degree) (Date) Title: LIFE HISTORY AND PRODUCTION STUDIES OF SIALIS CALIFORNICA BANKS AND SIALIS ROTUNDA BANKS (MEGALOPTERA:SIALIDAE) Abstract approved: Redacted for Privacy Norman H. Anderson Comparative ecological studies of Sialis californica Banks and Sialis rotunda Banks were made in Western Oregon from 1966 to 1968. Field collections for S. rotunda were made in fish holding pond at the Oak Creek laboratories, 5 miles west of Corvallis, Benton County, and for S. californica in enriched sections of Berry Creek experi- mental stream, 13 miles north of Corvallis. Life histories were determined in aquaria, in laboratory streams and the natural habitats.S. rotunda, predominantly a pond species, completed its life cycle in one year in all situations.S. californica, commonly a stream species, took one or two years depending on oviposition time and food availability.Both species were reared successfully in the laboratory through all stages of their life cycle.There are ten larval instars.The larvae are carnivorous and feed on insects and other small benthic organisms. Biweekly or monthly samples were collected from the two loca- tions for density, biomass, growth rate and production studies. Density and biomass of S. rotunda in the pond were much higher than for S. californica in the stream. Enrichment with sucrose and urea resulted in differences in density and biomass of S. californica between the four experimental sections at Berry Creek.The unenriched section usually had a high density but low or similar biomass compared with the enriched sections which had few individuals. -

Megaloptera, Sialidae)
MUSEUM & INSTITUTE OF ZOOLOGY POLISH ACADEMY OF SCIENCES FRAGMENTA FAUN I STIC A Fragm. faun. Warsaw, 30.12.2000 43 11 123-125 Wiesława C z e c h o w s k a Sialis morio K lingstedt, 1932 Megaloptera( , S ia lid a), e an alderfly species new to Poland Abstract: Sialis morio K lingstedt, 1932 is reported from Poland for the first time. It was found in two sites in the Masurian Lake District in the years 1998-1999. Key words:Neuropteroidea, Megaloptera , Sialis morio, Poland. Author's address: Museum and Institute of Zoology, PAS, Wilcza 64, 00-679 Warszawa, POLAND The Megaloptera is a small order of insects of the superorder Neuropteroi dea whose larval development occurs in an aquatic habitat. In Europe, this taxon is represented by 10 species of the genus Sialis L a t r . , the family Siali dae (A s p ó c k et al. 1980, V s h iv k o v a 1985, 1987). However, according to A s p ó c k (1992) and A s p ó c k and H o l z e l (1994), this genus should be revised, for some of the recently described species may be synonyms of others. The species considered unquestionable by these authors include Sialis lutaria L., S. morio K l i n g s t . , S. sordida K l i n g s t . , S. fuliginosa PICT., S. rtigripes PICT, and S. sibirica M c L a c h l . Three of these have been recorded from Poland, namely S. -
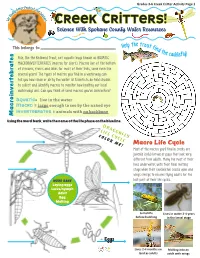
Creek Critters!
Grades 3-6 Creek Critter Activity Page 1 ter Protect Ot Ou lly r W ea a R t e e r W ! Creek Critters! Science With Spokane County Water Resources Help the trout f This belongs to: ind the ca ly! Fish, like the Redband Trout, eat aquatic bugs known as AQUATIC ddisf MACROINVERTEBRATES (macros for short). Macros live at the bottom of streams, rivers and lakes for most of their lives; some even live several years! The types of macros you find in a waterway can tell you how clean or dirty the water is! Scientists do field studies to collect and identify macros to monitor how healthy our local waterways are. Can you think of some macros you’ve seen before? Aquatic= live in the water Macro = large enough to see by the naked eye invertebrates = animals with no backbone Macroinvertebrates Using the word bank, write the name of the life phase on the blue line. DRAGONFLY LIFE CYCLE COLOR ME! Macro Life Cycle Most of the macros you’ll find in creeks are juvenile (child) larvae or pupa that look very different from adults. Many live most of their lives underwater, until their final molting stage when their exoskeleton cracks open and wings emerge to become flying adults for the Word Bank last part of their life cycles. Laying eggs Larva/nymph Adult Egg Molting 6 months Lives in water 3-4 years before hatching in the larval stage Eggs Lives 2-4 months on Molting into an land as adults adult with wings Grades 3-6 Creek Critter Activity Page 2 Table Manners! Freshwater Food Chain Macros have specialized mouth pieces to help them gather food or hunt. -

Arvalis Ross, S. Californica Banks, S. Cornuta Ross, S. Hamata Ross, S
AN ABSTRACT OF THE THESIS OF ELWIN D. EVANS for the DOCTOR OF PHILOSOPHY (Name) (Degree) in ENTOMOLOGY presented on October 4, 1971 (Major) (Date) Title: A STUDY OF THE MEGALOPTERA OF THE PACIFIC COASTAL REGION ,Or THE UNtjT5D STATES Abstract approved: N. H. /Anderson Nineteen species of Megaloptera occurring in the western United States and Canada were studied.In the Sialidae, the larvae of Sialis arvalis Ross, S. californica Banks, S. cornuta Ross, S. hamata Ross, S. nevadensis Davis, S. occidens Ross and S. rotunda Banks are described with a key for their identification.The female of S. arvalis is described for the first time.Descriptions of the egg masses, hatching, and the egg bursters and first instar larvae are givenfor some species.Data are given on larval habitats, life cycles, distribution and emergence of the adults. An evolutionaryscheme for the Sialidae in the study area and the world genera ishypothesized. In the Corydalidae, Orohermes gen. nov. andProtochauliodes cascadiusse.nov. are described.The adults of Corydalus cognatus Hagen, Dysmicohermes disjunctus Munroe, D. ingens Chandler, Orohermes crepusculus (Chandler), Neohermesfilicornis (Banks), N. californicus (Walker), Protochauliodes aridus Maddux, P. spenceri Munroe, P. montivagus.Chandler, P. simplus Chandler, and P. minimus (Davis) are also described.The larvae of all but three species are described.Keys are presented for identifying the adults and larvae.Egg masses, egg bursters and the mating behavior are given for some species.Pre-genital scent glands were found in the males of the Corydalidae.Data are given on the larval habitats, distribution and adult emergence.Life cycles of five years are estimated for some intermittent stream inhabitants and the cold stream species, 0. -

Phylogeny of Endopterygote Insects, the Most Successful Lineage of Living Organisms*
REVIEW Eur. J. Entomol. 96: 237-253, 1999 ISSN 1210-5759 Phylogeny of endopterygote insects, the most successful lineage of living organisms* N iels P. KRISTENSEN Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen 0, Denmark; e-mail: [email protected] Key words. Insecta, Endopterygota, Holometabola, phylogeny, diversification modes, Megaloptera, Raphidioptera, Neuroptera, Coleóptera, Strepsiptera, Díptera, Mecoptera, Siphonaptera, Trichoptera, Lepidoptera, Hymenoptera Abstract. The monophyly of the Endopterygota is supported primarily by the specialized larva without external wing buds and with degradable eyes, as well as by the quiescence of the last immature (pupal) stage; a specialized morphology of the latter is not an en dopterygote groundplan trait. There is weak support for the basal endopterygote splitting event being between a Neuropterida + Co leóptera clade and a Mecopterida + Hymenoptera clade; a fully sclerotized sitophore plate in the adult is a newly recognized possible groundplan autapomorphy of the latter. The molecular evidence for a Strepsiptera + Díptera clade is differently interpreted by advo cates of parsimony and maximum likelihood analyses of sequence data, and the morphological evidence for the monophyly of this clade is ambiguous. The basal diversification patterns within the principal endopterygote clades (“orders”) are succinctly reviewed. The truly species-rich clades are almost consistently quite subordinate. The identification of “key innovations” promoting evolution -

Chapter X —Order Megaloptera
Chapter X —Order Megaloptera (Alderflies- , Dobsonflies- , Fishflies- ) • (Williams & Feltmate, 1992) • Superphylum Arthropoda • (jointed-legged metazoan animals [Gr, arthron = joint; pous = foot]) • Phylum Entoma • Subphylum Uniramia • (L, unus = one; ramus = branch, referring to the unbranched nature of the ap- pendages) • Superclass Hexapoda • (Gr, hex = six, pous = foot) • Class Insecta • (L, insectum meaning cut into sections) • Subclass Ptilota • Infraclass Neopterygota The order Megaloptera is a small order of insects in the infraclass Neoptera, division Endoptery- gota. The Megaloptera are closely related to the Neuroptera (spongillaflies). The Megaloptera comprise only two families, the Corydalidae (fishflies and dobsonflies) and the Sialidae (alderflies). Larvae of all species of Megaloptera are aquatic and attain the largest size of all aquatic insects. Larval Corydalidae are sometimes called hellgrammites or toe biters. The adult Corydalidae are large, having a wing span of up to 16 cm (Megaloptera = “large wing”). Life History Females of this holometabolous order lay elongate eggs in masses on vegetation overhanging the aquatic habitat, on large rocks projecting from the water, or on bridge abutments. After about a week at cool temperatures, eggs hatch at night and first-instar larvae fall into the water. As young larvae swallow air, gas bubbles form in their guts, possibly providing the buoyancy neces- sary to transport to riffles first instars that land in pools. The metabolic consequences of this air bubble are unknown for most species. Megalopteran larvae go through 10-12 instars before crawling out of the water onto shore to pupate. Some have been reported to pupate as far as 50 metres from the shore. Bioassessment of Freshwaters using Benthic Macroinvertebrates- A Primer X-1 Most sialids have one- or two-year life cycles, whereas corydalids in cold mountain streams and in intermittent streams may live for up to five years. -

Biodiversity and Phenology of the Epibenthic Macroinvertebrate Fauna in a First Order Mississippi Stream
The University of Southern Mississippi The Aquila Digital Community Master's Theses Summer 8-2017 Biodiversity and Phenology of the Epibenthic Macroinvertebrate Fauna in a First Order Mississippi Stream Jamaal Bankhead University of Southern Mississippi Follow this and additional works at: https://aquila.usm.edu/masters_theses Recommended Citation Bankhead, Jamaal, "Biodiversity and Phenology of the Epibenthic Macroinvertebrate Fauna in a First Order Mississippi Stream" (2017). Master's Theses. 308. https://aquila.usm.edu/masters_theses/308 This Masters Thesis is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Master's Theses by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. BIODIVERSITY AND PHENOLOGY OF THE EPIBENTHIC MACROINVERTEBRATES FAUNA IN A FIRST ORDER MISSISSIPPI STREAM by Jamaal Lashwan Bankhead A Thesis Submitted to the Graduate School, the College of Science and Technology, and the Department of Biological Sciences at The University of Southern Mississippi in Partial Fulfillment of the Requirements for the Degree of Master of Science August 2017 BIODIVERSITY AND PHENOLOGY OF THE EPIBENTHIC MACROINVERTEBRATES FAUNA IN A FIRST ORDER MISSISSIPPI STREAM by Jamaal Lashwan Bankhead August 2017 Approved by: ________________________________________________ Dr. David C. Beckett, Committee Chair Professor, Biological Sciences ________________________________________________ Dr. Kevin Kuehn, Committee