Left-Sided Inferior Vena Cava with Hemiazygos Continuation to Left Superior Vena Cava
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Azygos Vein System Abnormality: Case Report
Gülhane Týp Dergisi 2006; 48: 180-182 OLGU SUNUMU © Gülhane Askeri Týp Akademisi 2006 Azygos vein system abnormality: case report Necdet Kocabýyýk (*), Tunç Kutoðlu (**), Soner Albay (*), Bülent Yalçýn (*), Hasan Ozan (*) Summary Introduction Variations seen in the thoracic vein system are Abnormalities related to the azygos system are not rare (1). In a series related to the development of these veins. of 200 cases, Bergman et al. have reported the incidence of this anomaly During the dissection from the posterior medi- astinum of the 60-year-old male cadaver, it 26% (2). These abnormalities are generally explained by the embryolog- was observed that there was no complete ical development. Venous branching of the azygos vein varies (3). There accessory hemiazygos vein, and both posterior are two origins of the azygos and hemiazygos veins. By union of these intercostal veins and hemiazygos vein (above origins and regression of some parts, azygos system comes into its final T10 level) drained bilaterally to the azygos vein. Considering these types of variations is status (4). Different types of structures may occur when these veins important during imaging this region and surgi- develop. Abnormalities about azygos system and especially the variations cal operations. of the hemiazygos veins are not clearly described in the literature. In this Key words: Azygos vein, hemiazygos vein, superior vena cava, venous anomaly presentation absence of the accessory hemiazygos vein and possible causes of these types of variations are discussed in view of the embry- Özet ological development. Azigos ven sistem anomalisi: olgu sunumu Toraks ven sisteminde görülen varyasyonlar, embriyolojik olarak bu venlerin geliþimiyle ilgi- Case Report lidir. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -
Endothelial Cell Dynamics in Vascular Development: Insights from Live-Imaging in Zebrafish
fphys-11-00842 July 20, 2020 Time: 12:10 # 1 REVIEW published: 22 July 2020 doi: 10.3389/fphys.2020.00842 Endothelial Cell Dynamics in Vascular Development: Insights From Live-Imaging in Zebrafish Kazuhide S. Okuda1,2 and Benjamin M. Hogan1,2,3* 1 Organogenesis and Cancer Program, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia, 2 Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, VIC, Australia, 3 Department of Anatomy and Neuroscience, University of Melbourne, Melbourne, VIC, Australia The formation of the vertebrate vasculature involves the acquisition of endothelial cell identities, sprouting, migration, remodeling and maturation of functional vessel networks. To understand the cellular and molecular processes that drive vascular development, live-imaging of dynamic cellular events in the zebrafish embryo have proven highly informative. This review focusses on recent advances, new tools and new insights from imaging studies in vascular cell biology using zebrafish as a model system. Keywords: vasculogenesis, angiogenesis, lymphangiogenesis, anastomosis, endothelial cell, zebrafish Edited by: INTRODUCTION Elizabeth Anne Vincent Jones, KU Leuven, Belgium Vascular development is an early and essential process in the formation of a viable vertebrate Reviewed by: embryo. Blood and lymphatic vascular networks are composed of a complex mix of cell types: for Anna Rita Cantelmo, example smooth muscle cells, fibroblasts, pericytes and immune cells are intimately associated and Université Lille Nord de France, even integrated within mature vessel walls (Rouget, 1873; Horstmann, 1952; Nicosia and Madri, France 1987; Fantin et al., 2010; Gordon et al., 2010). The inner lining of the vasculature, made up of Jingjing Zhang, endothelial cells (ECs), is the earliest part of the vasculature to develop in the embryo and is Affiliated Hospital of Guangdong Medical University, China instructive in recruiting other lineages and cell types as the vascular system matures. -

Development of the Vascular System in Five to Twenty-One
THE DEVELOPMENT OF THE VASCULAR SYSTEM IN FIVE TO TWtNTY-ONE SOMITE DOG EMBRYOS by ELDEN WILLIAM MARTIN B, S., Kansas State College of Agriculture and ADolied Science, 195>U A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Zoology KANSAS STATV: COLLEGE OF AGRICULTURE AND A PLIED SCIENCE 1958 LP TH Ooco/*>*Tv TABLE OF CONTENTS INTRO IXJ CTION AND LITERATURE REVIEW 1 MATERIALS AND METHODS ^ OBSERVATIONS 6 Five-Somi te Stag© . 6 Seven-Somite Stage 8 Eight-Somite Stage 9 Ten- and bleven-Somite Stage 12 Twe 1 ve-Somi te Stage • \\i Fifteen-Somite Stage 18 Seventeen-Somite Stage 21 Eighteen-Somite Stage 2$ Twenty- and Twenty- one -Somite Stage 27 INTERPRETATIONS AND DISCUSSION 30 Vasculogenesis • 30 Cardiogenesis 33 The Origin and Development of Arteries \ 3lj. Aortic Arches •••« 3I4. Cranial Arterie s ...•• 36 The Dorsal Aorta 37 Intersegmental AAteries 39 Vertebral Arteries 39 Vitelline Arteries }±q The Allantoic Artery \±\ Ill IITERPRETATION AND DISCUSSION (Contd.) The Origin and Development of Veins •• kl The Anterior Cardinal Veins . I4.I Posterior Cardinal Veins k2 Umbilical Veins U3 Common Cardinal Veins kh Interconnecting Vessels Ui> SUMMARY kl LITERA°URE CITED $1 ACKNOWLEDGMENTS 53 APPENDIX 5U HTmDUCTIOW AND LITFRATORF. rfvibw While the dog has been employed extensively as a labora- tory animal in various fields of scientific endeavour, the use of this animal in embryology has been neglected. As a con- sequence, the literature on the circulatory system of the dog was represented only by an unpublished thesis by Duffey (3) on oardlogenesis and the first heart movements. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -
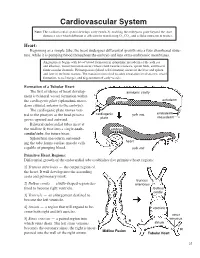
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

The Sinus Venosus Typeof Interatrial Septal Defect*
Thorax: first published as 10.1136/thx.13.1.12 on 1 March 1958. Downloaded from Thorax (I9%8), 13, 12. THE SINUS VENOSUS TYPE OF INTERATRIAL SEPTAL DEFECT* BY H. R. S. HARLEY Cardiff (RECEIVED FOR PUBLICATION DECEMBER 30, 1957) Defects of the interatrial septum, other than namely, (1) it lies above and independent of valvular patency of the foramen ovale, are often the fossa ovalis; (2) its margin is incomplete, classified into ostium primum and ostium secun- being absent superiorly and incomplete pos- dum varieties. The relationship of the former type teriorly; and (3) it is associated with anomalous to abnormal development of the atrioventricular drainage of the right superior, and sometimes of canal has been stressed by several workers, includ- the right middle or inferior, pulmonary vein. This ing Rogers and Edwards (1948), Wakai and type of defect is illustrated in Fig. 1 (after Lewis Edwards (1956), Wakai, Swan, and Wood (1956), et al., 1955) and Fig. 2 (after Geddes, 1912). In Brandenburg and DuShane (1956), Toscano- the case reported by Ross (1956), who kindly per- Barbosa, Brandenburg, and Burchell (1956), and mitted me to see the heart, the interatrial Cooley and Kirklin (1956). These workers prefer communication was described as ". lying the term "persistent common within the orifice of atrioventricular the superior vena cava in itscopyright. canal " to "persistent ostium primum." medial wall opposite the mouths of the anomalous In addition to the above types of interatrial pulmonary veins." Ross goes on to say: "On septal defect there is a third variety, which was casual inspection of the interior of the left atrium, described as long ago as 1868 by Wagstaffe, but the defect was not visible unless a search was made which has come into prominence only since the within the superior caval orifice." The relation- http://thorax.bmj.com/ introduction of surgical repair of interatrial ship of the defect to the orifice of the superior communications under direct vision. -

Cardiogenesis in the Bovine to 35 Somites
CARDIOGENESIS IN THE BOVINE TO 35 SOMITES by PATRICIA ANN NODEN A. A., Chanute Junior College, 1962 B. S., Kansas State University, 1964 A MASTER'S THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Zoology KANSAS STATE UNIVERSITY Manhattan, Kansas 1966 Approved by: ii N7G> TABLE OF CONTENTS Oocu mtA t INTRODUCTION 1 METHODS AND MATERIALS 6 OBSERVATIONS 9 Presomite Stage 9 Six Somite Stage 9 Nine Somite Stage 10 13 Somite Stage 18 19 Somite Stage 18 22 Somite Stage 19 24-25 Somite Stage 20 28-30 Somite Stage ....... 21 33-35 Somite Stage 24 DISCUSSION 27 CONCLUSION S3 ACKNOWLEDGMENTS 35 BIBLIOGRAPHY . 36 . INTRODUCTION Mammalian cardiogenesis is a vast field which so far has not been thoroughly explored. There are few species in which the complete development of the heart has been studied and many in which partial formation has been observed The formation of the heart to the functional stage in the dog (14 somites) was studied by Bonnet (1901) and Duffey (1953). Duffey's thesis was used exclusively for comparison in this paper. The rat has been studied in some detail, from presomite to birth and after, by Burlingame and Long (1939) and early stages by Ravn (1895) and Goss (1942, 1952). The tubular phase of cardiogenesis in the rabbit of 10-13 somites was described by Girgis (1930, 1933). Dwinnel (1939) worked with early somite stages. Development of the aortic arches was described by Bremer (1912). Hensen (1875), Bom (1888, 1889), Strahl and Carius (1889) and Rouviere (1904) have described early cardiac formation. -

Foxc1a and Foxc1b Differentially Regulate Angiogenesis from Arteries and Veins By
bioRxiv preprint doi: https://doi.org/10.1101/417931; this version posted September 14, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Running Title: foxc1a and foxc1b in angiogenesis Title: foxc1a and foxc1b differentially regulate angiogenesis from arteries and veins by modulating Vascular Endothelial Growth Factor signalling Authors: Zhen Jiang1,2, Teri Evans4, Aaron M. Savage1,2, Matthew Loose4, Timothy J.A. Chico1,2, Fredericus J.M. van Eeden2,3, Robert N. Wilkinson1,2 1 Department of Infection, Immunity and Cardiovascular Disease, Medical School, University of Sheffield, UK 2 The Bateson Centre, University of Sheffield, S10 2RX, UK 3 Department of Biomedical Science, University of Sheffield, S10 2TN, UK 4 School of Life Sciences, University of Nottingham, NG7 2UH, UK Corresponding author: Robert Wilkinson, Department of Infection, Immunity and Cardiovascular Disease, Medical School, University of Sheffield, S10 2RX, UK Email: [email protected] Key words: foxc1, angiogenesis, VEGF, Notch, zebrafish, blood vessel Summary Statement foxc1a and foxc1b promote angiogenesis from veins and suppress angiogenesis from arteries by promoting competing pro-angiogenic Vascular Endothelial Growth Factor signalling, and anti-angiogenic Dll4/Notch signalling in zebrafish embryos. bioRxiv preprint doi: https://doi.org/10.1101/417931; this version posted September 14, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Isl1-Expressing Non-Venous Cell Lineage Contributes to Cardiac Lymphatic Vessel Development
Developmental Biology 452 (2019) 134–143 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology Isl1-expressing non-venous cell lineage contributes to cardiac lymphatic vessel development Kazuaki Maruyama a, Sachiko Miyagawa-Tomita a,b,c, Kaoru Mizukami a, Fumio Matsuzaki d, Hiroki Kurihara a,e,* a Department of Physiological Chemistry and Metabolism, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan b Department of Pediatric Cardiology, Tokyo Women's Medical University, 8-1 Kawada-cho, Shinjuku-ku, Tokyo, 162-8666, Japan c Department of Animal Nursing Science, Yamazaki University of Animal Health Technology, 4-7-2 Minami-osawa, Hachioji, Tokyo, 192-0364, Japan d Laboratory for Cell Asymmetry, RIKEN Center for Developmental Biology, 2-2-3, Minatojiima-Minamimachi, Chuou-ku, Kobe, 650-0047, Japan e Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency (JST), Chiyoda-ku, Tokyo, 102-0076, Japan ABSTRACT The origin of the mammalian lymphatic vasculature has been studied for more than a century; however, details regarding organ-specific lymphatic development remain unknown. A recent study reported that cardiac lymphatic endothelial cells (LECs) stem from venous and non-venous origins in mice. Here, we identified Isl1- expressing progenitors as a potential non-venous origin of cardiac LECs. Genetic lineage tracing with Isl1-Cre reporter mice suggested a possible contribution from the Isl1-expressing pharyngeal mesoderm constituting the second heart field to lymphatic vessels around the cardiac outflow tract as well as to those in the facial skin and þ þ the lymph sac. -

Cardiovascular System - Accessscience from Mcgraw-Hill Education
Cardiovascular system - AccessScience from McGraw-Hill Education http://accessscience.com/content/109900 (http://accessscience.com/) Article by: Weichert, Charles K. College of Arts and Sciences, University of Cincinnati, Cincinnati, Ohio. Copenhaver, W. M. College of Physicians and Surgeons, Columbia University, New York; Department of Biological Structures, School of Medicine, University of Miami, Miami, Florida. Ebert, James D. Department of Embryology, Carnegie Institution, Washington, DC. Patten, Bradley M. Department of Anatomy, University of Michigan, Ann Arbor, Michigan. Jones, David R. Department of Zoology, University of British Columbia, Vancouver, Canada. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.109900 (http://dx.doi.org/10.1036/1097-8542.109900) Content Comparative Anatomy Embryogenesis of blood vessels Balancing ventricular output Heart Angiogenesis Human Postnatal Circulation Arterial system Circulatory system morphogenesis Pulmonary circuit and ductus Venous system Primitive venous system Physiological aspects of transition Comparative Embryology Functional Development of Heart Comparative Physiology Heart Contractions of the heart General physiology of circulation Tubular heart formation Heart-forming areas Microcirculation Cardiac loop and regional development Contractile proteins Heart Formation of definitive heart Synthesis of contractile proteins Arteries Partitioning of mammalian heart Action of inhibitors Venous system Division of atrium and ventricles Human Fetal Circulation at Term Bibliography -

Mafba Is a Downstream Transcriptional Effector of Vegfc Signaling Essential for Embryonic Lymphangiogenesis in Zebrafish
Downloaded from genesdev.cshlp.org on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press mafba is a downstream transcriptional effector of Vegfc signaling essential for embryonic lymphangiogenesis in zebrafish Katarzyna Koltowska,1 Scott Paterson,1 Neil I. Bower,1 Gregory J. Baillie,1 Anne K. Lagendijk,1 Jonathan W. Astin,2 Huijun Chen,1 Mathias Francois,1 Philip S. Crosier,2 Ryan J. Taft,1 Cas Simons,1 Kelly A. Smith,1 and Benjamin M. Hogan1 1Division of Genomics of Development and Disease, Institute for Molecular Bioscience, The University of Queensland, St Lucia, Brisbane, Queensland 4072, Australia; 2Department of Molecular Medicine and Pathology, School of Medical Sciences, University of Auckland, Auckland 1023, New Zealand The lymphatic vasculature plays roles in tissue fluid balance, immune cell trafficking, fatty acid absorption, cancer metastasis, and cardiovascular disease. Lymphatic vessels form by lymphangiogenesis, the sprouting of new lym- phatics from pre-existing vessels, in both development and disease contexts. The apical signaling pathway in lym- phangiogenesis is the VEGFC/VEGFR3 pathway, yet how signaling controls cellular transcriptional output remains unknown. We used a forward genetic screen in zebrafish to identify the transcription factor mafba as essential for lymphatic vessel development. We found that mafba is required for the migration of lymphatic precursors after their initial sprouting from the posterior cardinal vein. mafba expression is enriched in sprouts emerging from veins, and we show that mafba functions cell-autonomously during lymphatic vessel development. Mechanistically, Vegfc signaling increases mafba expression to control downstream transcription, and this regulatory relationship is de- pendent on the activity of SoxF transcription factors, which are essential for mafba expression in venous endothe- lium.