Molecules, Morphometrics and New Fossils Provide an Integrated View Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

103 the EVOLUTION of BATSI Leigh Van Valen Department Of
103 THE EVOLUTIONOF BATSI Leigh Van Valen Department of Biology University of Chicago 1103 East 57th Street Chicago, Illinois 60637 Received August 30, 1978; June 8, L979 Abstract: I devel-op the first explicitly Justified phylogeny of the known farnil-les of bats, usj.ng all available characters. This phylogeny permits the adaptive evolution of the order to be outlined. Two main clades emerge within the Microchiroptera and are cal-led new infraorders, Vespertilionia and Phyllostomatia. In each infraorder there is a series of grades of progressively stronger fi-ight, and there is a radiation of diet within the Phyllostomatia. Parallel evolution is extensive. Most grades stlll exist, presumably by a poorly understood partitioning of the resource space. The Megachiroptera nay have originated in the l-ate Ollgocene or early Miocene from surviving mernbers of the Eochiroptera (new suborder). The Kerivoul,ldae, Myzopodidae, Thyropteridae, and Furlpterldae are placed in the Natalidae, and the lcaronycterididae in the PaLaeochiropterygidae. The features of an ancestral bat are predicted. Bat origins are poorly known but may be found in Paleocene members of the Adapisoricidae. ,r** Bats constitute the second largest order of marnmalsand have a readily decipherable adaptive history, at least compared with the Rodentia and Insectivora. Nevertheless, there is no treatment of this adaptive hlstory nor even a general phyl-ogeny, which must form its foundation. I noticed this l-ack when revising a course on the paleobiology of mauunalsand undertook to remedy it. Although I sttll lack much familiarity with bats, the resul-t may be of more general interest. ORIGIN OF BATS One may hypothesize ttrat bats dld originate, but it is harder to go beyond this. -

New Miocene and Pliocene Megadermatids (Mammalia, Micro Chiroptera) from Australia, with Comments on Broader Aspects of Megadermatid Evolution
NEW MIOCENE AND PLIOCENE MEGADERMATIDS (MAMMALIA, MICRO CHIROPTERA) FROM AUSTRALIA, WITH COMMENTS ON BROADER ASPECTS OF MEGADERMATID EVOLUTION Suzar.nuJ.IIAND HAND S.J. 1996. New Miocene and Pliocene megadermatids (Mammalia, Microchiroptera) from Australia, with com- ments on broader aspects of megadermatid evolution. [Nouveaux M6gadermatid6s miocdnes et pliocbnes (Mammgliq, Microchiroptera) d'Australie;commentaires sur les aspects g6n6raux de l'6volution des M6gadermatid6sl. GEOBIOS' 29,3 :365-377. Villeurbanne le 30.06.1996. Manuscrit d6pos6 le 17.05.1993 ; accept6 d6finitivement le 10.01.1995. ABSTRACT - Macroderma malugara nov. sp., a new false vampire from the middle Miocene Gotham City Site of Riversleigh Station in northwestern Queensland, is described and three others discussed. Seven megadermatids are now known from the Australian fossil record. Morphological changes in the Australian Macroderma lineage are traced from the Oligo-Miocene to the present. In some Riversleigh deposits, there is evidence for sy'rnpatry of very differently-sized megadermatids. Riversleigh megadermatids provide an opportunity to trace an apparent trend to shorten the face in the Mairoclerma lineage flom the Oligo-Miocene to the present and to fufther document a tendency to gigantism in inde- pendent megadermatid lineages. KEY.WORDS : CHIROPTtrRA. NEW SPECIES, EVOLUTION, MIOCENE, PLIOCENE, AUSTRALIA RESUME - Macroderma malugara nov. sp.) un nouveau faux vampire du Miocdne moyen de Gotham City, Riversleigh Station, Queensland NW, est d6crit, et trois autres formes sont discut6es. Sept m6gadermatid6s fossiles sont maintenant connus d'Australie. Les modifications morphologiques dans la lign6e australienne de Macroderma sont retrac6es depuis l'Oligo-Miocbne jusqu'ir lActuel. Dans certains d6pdts de Riversleigh, la sSnnpatrie de m6gadermatid6s de diff6rentes tailles est 6vidente. -

New Early Eocene Mammalian Fauna from Western Patagonia, Argentina
Tejedor, M F; Goin, F J; Gelfo, J N; López, G; Bond, M; Carlini, A A; Scillato-Yané, G J; Woodburne, M O; Chornogubsky, L; Aragón, E; Reguero, M A; Czaplewski, N J; Vincon, S; Martin, G M; Ciancio, M R (2009). New Early Eocene mammalian fauna from western Patagonia, Argentina. American Museum Novitates, (3638):1-43. Postprint available at: http://www.zora.uzh.ch University of Zurich Posted at the Zurich Open Repository and Archive, University of Zurich. Zurich Open Repository and Archive http://www.zora.uzh.ch Originally published at: American Museum Novitates 2009, (3638):1-43. Winterthurerstr. 190 CH-8057 Zurich http://www.zora.uzh.ch Year: 2009 New Early Eocene mammalian fauna from western Patagonia, Argentina Tejedor, M F; Goin, F J; Gelfo, J N; López, G; Bond, M; Carlini, A A; Scillato-Yané, G J; Woodburne, M O; Chornogubsky, L; Aragón, E; Reguero, M A; Czaplewski, N J; Vincon, S; Martin, G M; Ciancio, M R Tejedor, M F; Goin, F J; Gelfo, J N; López, G; Bond, M; Carlini, A A; Scillato-Yané, G J; Woodburne, M O; Chornogubsky, L; Aragón, E; Reguero, M A; Czaplewski, N J; Vincon, S; Martin, G M; Ciancio, M R (2009). New Early Eocene mammalian fauna from western Patagonia, Argentina. American Museum Novitates, (3638):1-43. Postprint available at: http://www.zora.uzh.ch Posted at the Zurich Open Repository and Archive, University of Zurich. http://www.zora.uzh.ch Originally published at: American Museum Novitates 2009, (3638):1-43. PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, NY 10024 Number 3638, 43 pp., 10 figures, 1 table March 31, 2009 New Early Eocene Mammalian Fauna from Western Patagonia, Argentina MARCELO F. -

Observations, Discussions and Updates
African Bat Conservation News 2 May 2017 vol. 45 ISSN 1812-1268 Observations, Discussions and Updates RECENT CHANGES IN AFRicAN BAT TAXONOMY (2015 – 2017) VICTOR VAN CAKENBERGHE1,2 AND ERNest C.J. SEAMARK2,3 1 University of Antwerp, Department of Biology, Lab for Functional Morphology, Campus Drie Eiken, Universiteitsplein, 1, B-2610 Antwerpen (Wilrijk), Belgium. 2 AfricanBats NPC, 357 Botha Ave, Kloofsig, 0157. 3 Centre for Wildlife Management, University of Pretoria, Private Bag X20 Hatfield, Pretoria 0028, South Africa. †NECROMANTIDAE Sigé, 2011 was discovered. The specific epithet is given in honour of Mary Knight, Managing Editor of the American Museum of Natural History †Necromantis Weithofer, 1887 Scientific Publications, in recognition of the enormous contributions she has made to dissemination of the results of scientific research †Necromantis fragmentum Ravel, in Ravel, Adaci, over the years, as well as her lifelong devotion to the people and Bensalah, Charruault, Essid, AMmar, Marzougui, culture of Egypt. Mahboubi, Mebrouk, Merzeraud, Vianey-Liaud, Tabuce and Marivaux, 2016 Eidolinae Almeida, Giannini and Simmons, 2016 Locality and Horizon: Chambi (CBI) loci 1, 2 and 3, late lower This subfamily was created by ALMEIDA et al. (2016: 84) to Eocene - middle Eocene, Djebel Chambi, situated in the Kasserine distinguish monophyletic groups within the Pteropodidae. region, Tunisia. Currently recognized genera of Eidolinae: Eidolon Rafinesque, Etymology: From the Latin ‘fragmentum’, meaning fragment 1815. or debris, referring to the fragmentary character of the material attributed to this taxon. Pteropodinae Gray 1821 Subfamily arrangement based on ALMEIDA et al. (2016: 83). †AEGYPTONYCTERIDAE Simmons, Seiffert and Gunnell, Currently recognized genera of Pteropodinae: Acerodon Jourdan, 2016 1837, Macroglossus F. -

Megadermatidae: Macroderma) from the Kimberley Region of Western Australia
Records of the Australian Museum (2020) Records of the Australian Museum vol. 72, issue no. 5, pp. 161–174 a peer-reviewed open-access journal https://doi.org/10.3853/j.2201-4349.72.2020.1732 published by the Australian Museum, Sydney communicating knowledge derived from our collections ISSN 0067-1975 (print), 2201-4349 (online) A New Species of Extinct False Vampire Bat (Megadermatidae: Macroderma) from the Kimberley Region of Western Australia Kyle N. Armstrong1,2,3 , Ken Aplin1,4† , and Masaharu Motokawa1 1 The Kyoto University Museum, Yoshida Honmachi, Sakyo-ku, Kyoto, 606-8501, Japan 2 School of Biological Sciences, The University of Adelaide SA 5005, Australia 3 South Australian Museum, North Terrace, Adelaide SA 5000, Australia 4 Australian Museum Research Associate, 1 William Street, Sydney NSW 2010, Australia Abstract. A new species of False Vampire Bat (Megadermatidae), Macroderma handae sp. nov., is described from dental, dentary and maxillary fragments recovered from limestone deposits at Dingo Gap, Oscar Range, in the Kimberley region of Western Australia. This material is likely to be of Pliocene age, or early Pleistocene, based on biocorrelation within the same sample. The absence of the P2 indicates that it is more derived than Miocene taxa including M. malugara and M. godthelpi, but its phylogenetic position relative to M. koppa could not be determined. It appears to be slightly smaller than M. gigas and M. koppa 1 based on the size of M and M2. It can be distinguished from M. gigas by the lesser degree of fenestration in 1 the maxilla; and from all other species of Macroderma by the shape of the protofossa of the M , plus the M2 protoconid relatively high and of proportionally greater area within the trigonid. -

University of Michigan University Library
CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN VOL. 25, NO. 14, p. 277-287 (2 plates) December 31, 1981 LIPOTYPHLA, PROTEUTHERIA(?), AND CHIROPTERA (MAMMALIA) FROM THE EARLY-MIDDLE EOCENE KULDANA FORMATION OF KOHAT (PAKISTAN) BY DONALD E. RUSSELL and PHILIP D. GINGERICH MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN ANN ARBOR CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY Philip D. Gingerich, Director Gerald R. Smith, Editor This series of contributions from the Museum of Paleontology is a medium for the publica- tion of papers based chiefly upon the collection in the Museum. When the number of pages issued is sufficient to make a volume, a title page and a table of contents will be sent to libraries on the mailing list, and to individuals upon request. A list of the separate papers may also be obtained. Correspondence should be directed to the Museum of Paleontology, The University of Michigan, Ann Arbor, Michigan, 48109. VOLS. 11-XXV. Parts of volumes may be obtained if available. Price lists available upon inquiry. LIPOTYPHLA, PROTEUTHERIA(?), AND CHIROPTERA (MAMMALIA) FROM THE EARLY-MIDDLE EOCENE KULDANA FORMATION OF KOHAT (PAKISTAN) BY Donald E. Russell' and Philip D. Gingerich* Abstract.-Two new genera and species of insectivores, Seia shahi and Pakilestes lathrius, and an unnamed chiropteran are described from the early- middle Eocene Kuldana Formation at Chorlakki, Kohat District, North-West Frontier Province, Pakistan. These are the first insectivores and bats to be described from the Eocene of the Indo-Pakistan subcontinent. Seia appears to be an erinaceomorph lipotyphlan, but the affinities of Pakilestes are less certain. -
Macroderma Koppa, a New Tertiary Species of False Vampire Bat (Microchiroptera: Megadermatidae) from Wellington Caves, New South Wales
AUSTRALIAN MUSEUM SCIENTIFIC PUBLICATIONS Hand, Suzanne, Lyndall Dawson and M. Augee, 1988. Macroderma koppa, a new Tertiary species of false vampire bat (Microchiroptera: Megadermatidae) from Wellington Caves, New South Wales. Records of the Australian Museum 40(6): 343–351. [31 December 1988]. doi:10.3853/j.0067-1975.40.1988.160 ISSN 0067-1975 Published by the Australian Museum, Sydney naturenature cultureculture discover discover AustralianAustralian Museum Museum science science is is freely freely accessible accessible online online at at www.australianmuseum.net.au/publications/www.australianmuseum.net.au/publications/ 66 CollegeCollege Street,Street, SydneySydney NSWNSW 2010,2010, AustraliaAustralia Records of the Australian Museum (1988) Vol. 40: 343-351. ISSN 0067 1975 343 Macroderma koppa, a new Tertiary species of false vampire bat (Microchiroptera: Megadermatidae) from Wellington Caves, New South Wales SUZANNE HAND, LYNDALL DAWSON & MICHAEL AUGEE School of Zoology, University of New South Wales, Kensington, NSW 2033, Australia ABSTRACT. A new late Tertiary false vampire bat, Macroderma koppa, is described from the Big Sink doline of Wellington Caves, eastern central New South Wales. The new species appears to be the sister-group of the living Australian Ghost Bat, Macroderma gigas. Morphological features that distinguish the new species from M. gigas appear to be plesiomorphies shared with most other megadermatids. HAND, S., L. DAWSON & M. AUGEE, 1988. Macroderma koppa, a new Tertiary species offalse vampire bat (Microchiroptera: Megadermatidae) from Wellington Caves, New South Wales. Records of the Australian Museum 40( 6): 343-351. Until recently, all Australian fossil megadermatids Recent geological studies of the Wellington cave had been identified as conspecific with (or very close fills (Frank, 1971; Osborne, 1983) have indicated to) the only living Australian megadermatid, the their considerable stratigraphic complexity. -

African Bat Conservation News May 2017 ISSN 1812-1268
Volume 45 African Bat Conservation News May 2017 ISSN 1812-1268 © ECJ Seamark (AfricanBats NPC), 2016 Above: Bushveld Horseshoe Bat (Rhinolophus simulator) drinking from pond at the Meletse Bat Research and Conservation Training Centre, Limpopo Province, South Africa. Inside this issue Research and Conservation Activities Invitation to BAT1K Genome Consortium 2 Observations, Discussions and Updates Recent changes in African Bat Taxonomy (2015 – 2017) 2 Scientific contributions Update on the distribution of “Chiroptera sp1” in the southern and eastern parts of La Réunion Island based on acoustic surveys 5 Recent literature Notice Board Conferences 20 th 14 European Bat Research Symposium 20 th 4 Conference on Wind Energy and Wildlife Impacts 20 Southern African Bat Conference 20 th NASBR 47 Annual Symposium on Bat Research 20 Call for contributions 20 Download and subscribe to African Bat Conservation News published by AfricanBats at: www.africanbats.org The views and opinions expressed in articles are no necessarily those of the editor or publisher. Articles and news items appearing in African Bat Conservation News may be reprinted, provided the author’s and newsletter reference are given. African Bat Conservation News 2 May 2017 vol. 45 ISSN 1812-1268 Research and Conservation Activities INVITATION TO BAT1K GENOME CONSORTIUM EMMA TEELING, SONJA VERNES, DAVID RAY, LILIANA DAVALOS, TOM GILBERT AND GENE MYERS BAT1K is an new initiative aimed to sequence the genomes of all species, you can sign up at our website (bat1k.com), committing extant species of bats to chromosome level assemblies. Our goal to BAT1K and pledging these resources to the project. If you want as a consortium is to uncover the genes and genetic mechanisms to pledge other resources (i.e. -

First Record of the Genus Megaderma Geoffroy (Microchiroptera: Megadermatidae) from Australia
FIRST RECORD OF THE GENUS MEGADERMA GEOFFROY (MICROCHIROPTERA: MEGADERMATIDAE) FROM AUSTRALIA by Suzanne HAND * CONTENTS Page Abstract, Resume . 48 Introduction ..................................................................... 48 Systematics ..................................................................... 49 Comparisons with other megadermatids ............................................... 53 Fossil forms. 53 Living taxa. 55 Phylogenetic relationships. 57 Discussion ...................................................................... 58 Assignment to Megaderma . 58 Intrageneric relationships ....................................................... 60 Palaeobiogeography and palaeoecology . 61 Acknowledgements ............................................................... 62 References ...................................................................... 63 Appendix ....................................................................... 65 Legends of plates '" . .. 66 * School of Biological Science, University of New South Wales, PO Box I, Kensington, Sydney, NSW 2033, Australia Key-words: Chiroptera, Megademza, Megadermatidae, Pliocene, Rackham's Roost Site, Riversleigh, Australia. Mots-cles: Chiroptera, Megademza, Megadermatidae, Plioc<me, Localite Rackham's Roost, Riversleigh, Australie. Palaeoverlebrala, Montpellier, 24 (l~2): 47-66. 2 pI. (Rc~u Ie 29 Jumet 1993. accepte Ie 23 Septembre 1993, publie Ie 14 Juin 1995) ABSTRACT A new Tertiary megadennatid is described from Rackham's Roost Site, a Pliocene limestone cave deposit -

Catalogue of the Eocene Mammal Types of the Natural History Museum Basel
Loïc COSTEUR 1, 2 Martin SCHNEIDER 1 1: Naturhistorisches Museum Basel, Augustinergasse 2, 4001 Basel (Switzerland) 2: email: [email protected] ISBN 978-2-916733-10-4 Dépôt légal à parution Manuscript online since October 10, 2011 Carnets de Géologie (2011: Book 3 - Livre 3) Catalogue of the Eocene mammal types of the Natural History Museum Basel. Loïc COSTEUR & Martin SCHNEIDER Abstract This catalogue presents a list of the Eocene mammalian type specimens held in the collection of the Natural History Museum Basel (Natur- historisches Museum Basel, hereafter NMB). After a close inspection of the very rich Eocene collection (several tens of thousands of specimens), a total of 51 valid holotypes (plus 16 invalid species) were identified together with 21 valid lectotypes and several hundreds paratypes, paralectotypes and syntypes. These types belong to 123 originally described species, of which 99 are still valid, 30 having been moved to another genus. Introduction The vertebrate collection of the NMB is one of the most relevant of Europe regarding fossil mammals. The tradition of collecting and acquiring all kinds of fossil mammals dates back to the first curator for vertebrates at Basel, Ludwig RÜTIMEYER (1825-1895). He set the bases of this collection while collecting at various and now well-known Swiss localities. His famous successsor Hans Georg STEHLIN (1870-1941), father of the "Grande Coupu- re" (recognition of the Eocene-Oligocene faunal renewal), largely contribu- ted in popularizing the collection in the palaeontologcal community as he described several tens of species from the Swiss fossil record, and beyond, from the whole European record. -
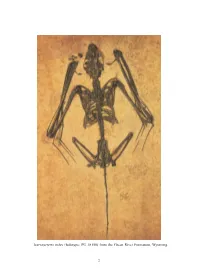
2 Icaronycteris Index
Icaronycteris index (holotype; PU 18150) from the Green River Formation, Wyoming. 2 CONTENTS Abstract ....................................................................... 4 Introduction .................................................................... 5 Relationships and Classi®cation of Eocene Bats: A Historical Overview ........... 12 Relationships Among Extant Lineages of Bats .................................. 31 Goals of the Current Study ................................................... 40 Materials and Methods ......................................................... 40 Taxonomic Sampling and Monophyly .......................................... 40 Outgroups .................................................................. 42 The Data Set ................................................................ 46 Characters Examined in Fossil Bats ............................................ 48 Skull and Dentition ........................................................ 48 Anterior Axial Skeleton .................................................... 64 Pectoral Girdle ............................................................ 70 Forelimb ................................................................. 77 Posterior Axial Skeleton and Pelvis .......................................... 81 Hindlimb ................................................................. 84 Completeness ............................................................... 85 Methods of Phylogenetic Analysis ............................................. 86 Analysis -

First Record of the Genus Megaderma Gpot .Roy (Microchiroptera
FIRST RECORD OF THE GENUS MEGADERMA GPOT.ROY (MICROCHIROPTERA : MEGADERMATIDAE) FROM AUSTRALIA by SuzanneHAND * CONTENTS Page Abstract,Rdsum6 48 Introduction 48 Systematics 49 Comparisons with other megadermatids . 53 Fossil forms 53 Living taxa. 55 Phylogeneticrelationships 57 Discussion 58 Assignment to M egaderma 58 Intrageneric relationships 60 Palaeobiogeographyand palaeoecology . 61 Acknowledgements 62 References 63 Appendix. .. 65 Legendsofplates 66 * School of Biological Science,University of New South Wales,PO Box l, Kensington,Sydney, NSW 2033, Australia Key-words: Chiroptera, Megaderma, Megadermatidae, Pliocene, Rackham's Roost Site, Riversleigh, Australia. Mots-cl6s: Chiroptera, Megaderma, Megadermatidae,PliocEne, Localit6 Rackham's Roost, Riversleigh, Australie. Paheovertebrara, Montpellier,24 (l-2): 4146,2 pl. (Regule 29Juillet 1993, acceptd le 23 Septembre1993, publi€ le 14Juin 1995) ABSTRACT A new Tertiary megadermatid is described from Rackham's Roost Site, a Pliocene limestone cave deposit on Riverileigh Station, northwestern Queensland, Australia. It appears to represent the first Australian record of Megaderma GeoFrnoy, 1810, a genus otherwise known from Tertiary African and (Lyroderma) European taxa and the living Asian species M. spasma (Lu'rNeeus,1758) and M- lyra perens, 1872. Megaderma richardsi n. sp. is one of the smallest megadermatids known. It exhibits a mixture of plesiomorphic and autapomorphic features, the latter appearing to exclude it from being from the ancestralto any living megadermatid.The new speciesis one of eight megadermatidsidentified Australian fossil record, most of which are referable to Macroderma Mtu-en, 1906' RESUME gisement Un nouveau mdgadermatid6 tertiaire est ddcrit de la localit6 Rackham's Roost, un premidre cavernicolepliocbne de RiversleighStation, Nord-Ouest Queensland, Australie' Il repr6sentela jusqu'ici decouverteaustralienne du Megaderma GeorrRov, 1810, un genre connu par des espdces et tertiairesafricaines et europdenneset par les espbcesrdcentes asiatiques M.