Fusion Tags for Protein Solubility, Purification, And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Elimination Pathways of Fusion Protein and Peptide Drugs
Review Volume 11, Issue 2, 2021, 9139 - 9147 https://doi.org/10.33263/BRIAC112.91399147 Elimination Pathways of Fusion Protein and Peptide Drugs Ali Khodadoust 1 , Hossein Aghamollaei 2 , Ali Mohammad Latifi 1 , Kazem Hasanpour 3 , Mahdi Kamali 4 , Hamid Tebyanian 5 , Gholamreza Farnoosh 1,* 1 Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran 2 Chemical Injuries Research Center, Systems biology and Poisonings Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran 3 Sabzevar University of Medical Sciences, School of Medicine, Sabzevar, Iran 4 Nanobiotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran 5 Research Center for Prevention of Oral and Dental Diseases, Baqiyatallah University of Medical Sciences, Tehran, Iran * Correspondence: [email protected]; Scopus Author ID 55855454400 Received: 28.07.2020; Revised: 25.08.2020; Accepted: 27.08.2020; Published: 1.09.2020 Abstract: Fusion proteins have been known as an interesting subject for scientific researches in improving properties or making a new function by synergistically incorporating two protein domains into one complex. Fusion proteins are created by ligation of two or more protein domains in a single polypeptide with functional properties. Improvement of therapeutic function is one of the main goals for developing these products. Elimination from the body is one of the most important points that should be considered in the design and production of fusion proteins. This review describes some of the most important excretion/elimination pathways of fusion peptide and protein drugs as well as serious elimination challenges in the development and manufacturing of fusion proteins. Keywords: Fusion proteins; Therapy; Pharmacokinetics; Serum half-life; Peptide. -

Understanding and Exploiting Post-Translational Modifications for Plant Disease Resistance
biomolecules Review Understanding and Exploiting Post-Translational Modifications for Plant Disease Resistance Catherine Gough and Ari Sadanandom * Department of Biosciences, Durham University, Stockton Road, Durham DH1 3LE, UK; [email protected] * Correspondence: [email protected]; Tel.: +44-1913341263 Abstract: Plants are constantly threatened by pathogens, so have evolved complex defence signalling networks to overcome pathogen attacks. Post-translational modifications (PTMs) are fundamental to plant immunity, allowing rapid and dynamic responses at the appropriate time. PTM regulation is essential; pathogen effectors often disrupt PTMs in an attempt to evade immune responses. Here, we cover the mechanisms of disease resistance to pathogens, and how growth is balanced with defence, with a focus on the essential roles of PTMs. Alteration of defence-related PTMs has the potential to fine-tune molecular interactions to produce disease-resistant crops, without trade-offs in growth and fitness. Keywords: post-translational modifications; plant immunity; phosphorylation; ubiquitination; SUMOylation; defence Citation: Gough, C.; Sadanandom, A. 1. Introduction Understanding and Exploiting Plant growth and survival are constantly threatened by biotic stress, including plant Post-Translational Modifications for pathogens consisting of viruses, bacteria, fungi, and chromista. In the context of agriculture, Plant Disease Resistance. Biomolecules crop yield losses due to pathogens are estimated to be around 20% worldwide in staple 2021, 11, 1122. https://doi.org/ crops [1]. The spread of pests and diseases into new environments is increasing: more 10.3390/biom11081122 extreme weather events associated with climate change create favourable environments for food- and water-borne pathogens [2,3]. Academic Editors: Giovanna Serino The significant estimates of crop losses from pathogens highlight the need to de- and Daisuke Todaka velop crops with disease-resistance traits against current and emerging pathogens. -

Ginkgolic Acid, a Sumoylation Inhibitor, Promotes Adipocyte
www.nature.com/scientificreports OPEN Ginkgolic acid, a sumoylation inhibitor, promotes adipocyte commitment but suppresses Received: 25 October 2017 Accepted: 15 January 2018 adipocyte terminal diferentiation Published: xx xx xxxx of mouse bone marrow stromal cells Huadie Liu1,2, Jianshuang Li2, Di Lu2, Jie Li1,2, Minmin Liu 3, Yuanzheng He4, Bart O. Williams2, Jiada Li1 & Tao Yang 2 Sumoylation is a post-translational modifcation process having an important infuence in mesenchymal stem cell (MSC) diferentiation. Thus, sumoylation-modulating chemicals might be used to control MSC diferentiation for skeletal tissue engineering. In this work, we studied how the diferentiation of mouse bone marrow stromal cells (mBMSCs) is afected by ginkgolic acid (GA), a potent sumoylation inhibitor also reported to inhibit histone acetylation transferase (HAT). Our results show that GA promoted the diferentiation of mBMSCs into adipocytes when cultured in osteogenic medium. Moreover, mBMSCs pre-treated with GA showed enhanced pre-adipogenic gene expression and were more efciently diferentiated into adipocytes when subsequently cultured in the adipogenic medium. However, when GA was added at a later stage of adipogenesis, adipocyte maturation was markedly inhibited, with a dramatic down-regulation of multiple lipogenesis genes. Moreover, we found that the efects of garcinol, a HAT inhibitor, difered from those of GA in regulating adipocyte commitment and adipocyte maturation of mBMSCs, implying that the GA function in adipogenesis is likely through its activity as a sumoylation inhibitor, not as a HAT inhibitor. Overall, our studies revealed an unprecedented role of GA in MSC diferentiation and provide new mechanistic insights into the use of GA in clinical applications. -

CUE-101, a Novel Fc Fusion Protein for Selective Targeting and Expansion of Anti-Tumor T Cells for Treatment of HPV-Driven Malignancies
CUE-101, a novel Fc fusion protein for selective targeting and expansion of anti-tumor T cells for treatment of HPV-driven malignancies Natasha Girgis1, Steven N. Quayle1, Alyssa Nelson1, Dharma Raj Thapa1, Sandrine Hulot1, Lauren Kraemer1, Miguel Moreta1, Zohra Merazga1, Robert Ruidera1, Dominic Beal1, Mark Haydock1, Jonathan Soriano1, Luke Witt1, Jessica Ryabin1, Emily Spaulding1, John F. Ross1, Saso Cemerski1, Anish Suri1, Rodolfo Chaparro1, Ronald Seidel1, Kenneth J. Pienta2, Mary C. Simcox1 1Cue Biopharma, Cambridge, Massachusetts; 2The James Buchanan Brady Urological Institute and the Department of Urology, Johns Hopkins School of Medicine, Baltimore, Maryland + + Background CUE-101 selectively expands E711-20-specific CD8 T mCUE-101 expands functional antigen-specific CD8 T • Human papilloma virus (HPV) is responsible for 72% of oropharyngeal, 90% of cervical, 90% cells from healthy human PBMCs cells in the tumor and the periphery of anal, and 71% of vulvar, vaginal, or penile cancers, causing significant morbidity and A. B. A. B. C. 9 mortality worldwide. Innovative therapies are urgently needed for these malignancies, Vehicle 100 nM CUE-101 107 15 Peripheral Blood particularly in the largely incurable metastatic setting. 106 10 5 • The E7 oncoprotein is constitutively expressed in HPV-associated cancers, is necessary for 105 ⍺PD-1 5 PE 4 Vehicle mCUE-101 Combo - 10 initiation and maintenance of malignant transformation, and is genetically conserved in 1 cancer (Mirabello 2017). 103 1 1 102 E7-Specific of CD8+ T cells % IFNg+,TNFa+ -

Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation
Review Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation Leonor S. Castro 1,†, Guilherme S. Lobo 1,†, Patrícia Pereira 2 , Mara G. Freire 1 ,Márcia C. Neves 1,* and Augusto Q. Pedro 1,* 1 CICECO–Aveiro Institute of Materials, Chemistry Department, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal; [email protected] (L.S.C.); [email protected] (G.S.L.); [email protected] (M.G.F.) 2 Centre for Mechanical Engineering, Materials and Processes, Department of Chemical Engineering, University of Coimbra, Rua Sílvio Lima-Polo II, 3030-790 Coimbra, Portugal; [email protected] * Correspondence: [email protected] (M.C.N.); [email protected] (A.Q.P.) † These authors contributed equally to this work. Abstract: The advent of biopharmaceuticals in modern medicine brought enormous benefits to the treatment of numerous human diseases and improved the well-being of many people worldwide. First introduced in the market in the early 1980s, the number of approved biopharmaceutical products has been steadily increasing, with therapeutic proteins, antibodies, and their derivatives accounting for most of the generated revenues. The success of pharmaceutical biotechnology is closely linked with remarkable developments in DNA recombinant technology, which has enabled the production of proteins with high specificity. Among promising biopharmaceuticals are interferons, first described by Isaacs and Lindenmann in 1957 and approved for clinical use in humans nearly thirty years later. Interferons are secreted autocrine and paracrine proteins, which by regulating several biochemical Citation: Castro, L.S.; Lobo, G.S.; pathways have a spectrum of clinical effectiveness against viral infections, malignant diseases, Pereira, P.; Freire, M.G.; Neves, M.C.; and multiple sclerosis. -
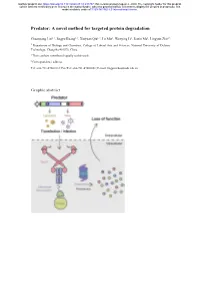
A Novel Method for Targeted Protein Degradation
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.31.231787; this version posted August 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Predator: A novel method for targeted protein degradation Chuanyang Liu1, †, Jingyu Kuang1, †, Xinyuan Qiu1, †, Lu Min1, Wenying Li1, Jiaxin Ma1, Lingyun Zhu1,* 1 Department of Biology and Chemistry, College of Liberal Arts and Sciences, National University of Defense Technology, Changsha 410073, China † These authors contributed equally to this work. ∗ Correspondence address. Tel: +86-731-87001812; Fax/Tel: +86-731-87001801; E-mail: [email protected] Graphic abstract bioRxiv preprint doi: https://doi.org/10.1101/2020.07.31.231787; this version posted August 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Protein expression and degradation are fundamental to cell function and physiological status of organisms. Interfering with protein expression not only provides powerful strategies to analyze the function of proteins but also inspires effective treatment methods for diseases caused by protein dysfunction. Recently, harnessing the power of the ubiquitin-proteasome system for targeted protein degradation (TPD) has become the focus of researches. Over the past two decades, TPD technologies, such as E3 ligase modification, PROTACs, and the Trim-Away method, have successfully re-oriented the ubiquitin-proteasome pathway and thus degraded many pathogenic proteins and even "undruggable" targets. -

Overview of Antibody Drug Delivery
pharmaceutics Review Overview of Antibody Drug Delivery Sahar Awwad 1,2,* ID and Ukrit Angkawinitwong 1 1 UCL School of Pharmacy, London WC1N 1AX, UK; [email protected] 2 National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, London EC1 V9EL, UK * Correspondence: [email protected]; Tel.: +44-207-753-5802 Received: 27 March 2018; Accepted: 29 June 2018; Published: 4 July 2018 Abstract: Monoclonal antibodies (mAbs) are one of the most important classes of therapeutic proteins, which are used to treat a wide number of diseases (e.g., oncology, inflammation and autoimmune diseases). Monoclonal antibody technologies are continuing to evolve to develop medicines with increasingly improved safety profiles, with the identification of new drug targets being one key barrier for new antibody development. There are many opportunities for developing antibody formulations for better patient compliance, cost savings and lifecycle management, e.g., subcutaneous formulations. However, mAb-based medicines also have limitations that impact their clinical use; the most prominent challenges are their short pharmacokinetic properties and stability issues during manufacturing, transport and storage that can lead to aggregation and protein denaturation. The development of long acting protein formulations must maintain protein stability and be able to deliver a large enough dose over a prolonged period. Many strategies are being pursued to improve the formulation and dosage forms of antibodies to improve efficacy and to increase the range of applications for the clinical use of mAbs. Keywords: antibodies; protein; pharmacokinetics; drug delivery; stability 1. -

Sumoylation and Phosphorylation Cross-Talk in Hepatocellular Carcinoma
Review Article SUMOylation and phosphorylation cross-talk in hepatocellular carcinoma Maria Lauda Tomasi, Komal Ramani Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA Contributions: (I) Conception and design: All authors; (II) Administrative support: All authors; (III) provision of study materials or patients: All authors; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Maria Lauda Tomasi, PhD; Komal Ramani, PhD. Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA. Email: [email protected]; [email protected]. Abstract: Hepatocellular carcinoma (HCC) is a primary malignancy of the liver and occurs predominantly in patients with underlying chronic liver disease and cirrhosis. The large spectrum of protein post- translational modification (PTM) includes numerous critical signaling events that occur during neoplastic transformation. PTMs occur to nearly all proteins and increase the functional diversity of proteins. We have reviewed the role of two major PTMs, SUMOylation and phosphorylation, in the altered signaling of key players in HCC. SUMOylation is a PTM that involves addition of a small ubiquitin-like modifiers (SUMO) group to proteins. It is known to regulate protein stability, protein-protein interactions, trafficking and transcriptional activity. The major pathways that are regulated by SUMOylation and may influence HCC are regulation of transcription, cell growth pathways associated with B-cell lymphoma 2 (Bcl-2) and methionine adenosyltransferases (MAT), oxidative stress pathways [nuclear erythroid 2-related factor 2 (Nrf2)], tumor suppressor pathways (p53), hypoxia-inducible signaling [hypoxia-inducible factor-1 (HIF-1)], glucose and lipid metabolism, nuclear factor kappa B (NF-κB) and β-Catenin signaling. -

The Cycle of Protein Engineering: Bioinformatics Design of Two Dimeric Proteins and Computational Design of a Small Globular Domain
The Cycle of Protein Engineering: Bioinformatics Design of Two Dimeric Proteins and Computational Design of a Small Globular Domain DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Venuka Durani, M.Sc. Graduate Program in Chemistry The Ohio State University 2012 Dissertation Committee: Thomas J. Magliery, Advisor Ross E. Dalbey Karin Musier-Forsyth William C. Ray Copyright by Venuka Durani 2012 Abstract The protein folding problem is an ongoing challenge, and even though there have been significant advances in our understanding of proteins, accurately predicting the effect of amino acid mutations on the structure and stability of a protein remains a challenge. This makes the task of engineering proteins to suit our purposes labor intensive as significant trial and error is involved. In this thesis, we have explored possibilities of better understanding and if possible improving some bioinformatics and computational methods to study proteins and also to engineer them. A significant portion of this thesis is based on bioinformatics approaches involving consensus and correlation analyses of multiple sequence alignments. We have illustrated how consensus and correlation metrics can be calculated and analyzed to explore various aspects of protein structure. Proteins triosephosphate isomerase and Cu, Zn superoxide dismutase were studied using these approaches and we found that a significant amount of information about a protein fold is encoded at the consensus level; however, the effect of amino acid correlations, while subtle, is significant nonetheless and some of the failures of consensus approach can be attributed to broken amino acid correlations. -

Heat Shock Protein 27 Is Involved in SUMO-2&Sol
Oncogene (2009) 28, 3332–3344 & 2009 Macmillan Publishers Limited All rights reserved 0950-9232/09 $32.00 www.nature.com/onc ORIGINAL ARTICLE Heat shock protein 27 is involved in SUMO-2/3 modification of heat shock factor 1 and thereby modulates the transcription factor activity M Brunet Simioni1,2, A De Thonel1,2, A Hammann1,2, AL Joly1,2, G Bossis3,4,5, E Fourmaux1, A Bouchot1, J Landry6, M Piechaczyk3,4,5 and C Garrido1,2,7 1INSERM U866, Dijon, France; 2Faculty of Medicine and Pharmacy, University of Burgundy, Dijon, Burgundy, France; 3Institut de Ge´ne´tique Mole´culaire UMR 5535 CNRS, Montpellier cedex 5, France; 4Universite´ Montpellier 2, Montpellier cedex 5, France; 5Universite´ Montpellier 1, Montpellier cedex 2, France; 6Centre de Recherche en Cance´rologie et De´partement de Me´decine, Universite´ Laval, Quebec City, Que´bec, Canada and 7CHU Dijon BP1542, Dijon, France Heat shock protein 27 (HSP27) accumulates in stressed otherwise lethal conditions. This stress response is cells and helps them to survive adverse conditions. We have universal and is very well conserved through evolution. already shown that HSP27 has a function in the Two of the most stress-inducible HSPs are HSP70 and ubiquitination process that is modulated by its oligomeriza- HSP27. Although HSP70 is an ATP-dependent chaper- tion/phosphorylation status. Here, we show that HSP27 is one induced early after stress and is involved in the also involved in protein sumoylation, a ubiquitination- correct folding of proteins, HSP27 is a late inducible related process. HSP27 increases the number of cell HSP whose main chaperone activity is to inhibit protein proteins modified by small ubiquitin-like modifier aggregation in an ATP-independent manner (Garrido (SUMO)-2/3 but this effect shows some selectivity as it et al., 2006). -

COVID-19: Mechanisms of Vaccination and Immunity
Review COVID-19: Mechanisms of Vaccination and Immunity Daniel E. Speiser 1,* and Martin F. Bachmann 2,3,4,* 1 Department of Oncology, University Hospital and University of Lausanne, 1066 Lausanne, Switzerland 2 International Immunology Centre, Anhui Agricultural University, Hefei 230036, China 3 Department of Rheumatology, Immunology and Allergology, Inselspital, University of Bern, 3010 Bern, Switzerland 4 Department of BioMedical Research, University of Bern, 3008 Bern, Switzerland * Correspondence: [email protected] (D.E.S.); [email protected] (M.F.B.) Received: 2 July 2020; Accepted: 20 July 2020; Published: 22 July 2020 Abstract: Vaccines are needed to protect from SARS-CoV-2, the virus causing COVID-19. Vaccines that induce large quantities of high affinity virus-neutralizing antibodies may optimally prevent infection and avoid unfavorable effects. Vaccination trials require precise clinical management, complemented with detailed evaluation of safety and immune responses. Here, we review the pros and cons of available vaccine platforms and options to accelerate vaccine development towards the safe immunization of the world’s population against SARS-CoV-2. Favorable vaccines, used in well-designed vaccination strategies, may be critical for limiting harm and promoting trust and a long-term return to normal public life and economy. Keywords: SARS-CoV-2; COVID-19; nucleic acid tests; serology; vaccination; immunity 1. Introduction The COVID-19 pandemic holds great challenges for which the world is only partially prepared [1]. SARS-CoV-2 combines serious pathogenicity with high infectivity. The latter is enhanced by the fact that asymptomatic and pre-symptomatic individuals can transmit the virus, in contrast to SARS-CoV-1 and MERS-CoV, which were transmitted by symptomatic patients and could be contained more efficiently [2,3]. -

Incubation Period of Measles from Exposure to Prodrome ■ Exposure to Rash Onset Averages 11 to 12 Days
Measles Paul Gastanaduy, MD; Penina Haber, MPH; Paul A. Rota, PhD; and Manisha Patel, MD, MS Measles is an acute, viral, infectious disease. References to measles can be found from as early as the 7th century. The Measles disease was described by the Persian physician Rhazes in the ● Acute viral infectious disease 10th century as “more to be dreaded than smallpox.” ● First described in 7th century In 1846, Peter Panum described the incubation period of ● Vaccines first licensed include measles and lifelong immunity after recovery from the disease. measles in 1963, MMR in 1971, John Enders and Thomas Chalmers Peebles isolated the virus in and MMRV in 2005 human and monkey kidney tissue culture in 1954. The first live, ● Infection nearly universal attenuated vaccine (Edmonston B strain) was licensed for use in during childhood in the United States in 1963. In 1971, a combined measles, mumps, prevaccine era and rubella (MMR) vaccine was licensed for use in the United ● Still common and often fatal States. In 2005, a combination measles, mumps, rubella, and in developing countries varicella (MMRV) vaccine was licensed. Before a vaccine was available, infection with measles virus was nearly universal during childhood, and more than 90% of persons were immune due to past infection by age 15 years. Measles is still a common and often fatal disease in developing countries. The World Health Organization estimates there were 142,300 deaths from measles globally in 2018. In the United 13 States, there have been recent outbreaks; the largest occurring in 2019 primarily among people who were not vaccinated.