The Decline and Conservation Management of the Threatened Endemic Palms of the Mascarene Islands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Approved Plant List 10/04/12
FLORIDA The best time to plant a tree is 20 years ago, the second best time to plant a tree is today. City of Sunrise Approved Plant List 10/04/12 Appendix A 10/4/12 APPROVED PLANT LIST FOR SINGLE FAMILY HOMES SG xx Slow Growing “xx” = minimum height in Small Mature tree height of less than 20 feet at time of planting feet OH Trees adjacent to overhead power lines Medium Mature tree height of between 21 – 40 feet U Trees within Utility Easements Large Mature tree height greater than 41 N Not acceptable for use as a replacement feet * Native Florida Species Varies Mature tree height depends on variety Mature size information based on Betrock’s Florida Landscape Plants Published 2001 GROUP “A” TREES Common Name Botanical Name Uses Mature Tree Size Avocado Persea Americana L Bahama Strongbark Bourreria orata * U, SG 6 S Bald Cypress Taxodium distichum * L Black Olive Shady Bucida buceras ‘Shady Lady’ L Lady Black Olive Bucida buceras L Brazil Beautyleaf Calophyllum brasiliense L Blolly Guapira discolor* M Bridalveil Tree Caesalpinia granadillo M Bulnesia Bulnesia arboria M Cinnecord Acacia choriophylla * U, SG 6 S Group ‘A’ Plant List for Single Family Homes Common Name Botanical Name Uses Mature Tree Size Citrus: Lemon, Citrus spp. OH S (except orange, Lime ect. Grapefruit) Citrus: Grapefruit Citrus paradisi M Trees Copperpod Peltophorum pterocarpum L Fiddlewood Citharexylum fruticosum * U, SG 8 S Floss Silk Tree Chorisia speciosa L Golden – Shower Cassia fistula L Green Buttonwood Conocarpus erectus * L Gumbo Limbo Bursera simaruba * L -
Winter-Fall Sale 2002 Palm Trees-Web
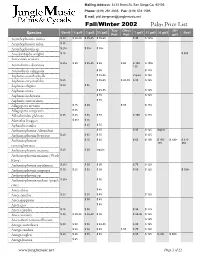
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

Saving Tahina — You Can Help
July 2020 NEWSLETTER SAVING TAHINA — YOU CAN HELP PalmTalk (the public forum of the International Palm Society) is initiating a pro- gram to preserve Tahina spectabilis, an endangered palm that PalmTalk brought to the world’s attention in 2007. The discovery of this enormous, new palm gen- erated worldwide interest, but now due to its extremely limited and fragile range in Madagascar, Tahina needs our help. Please visit PalmTalk (go to www. palms.org, and click on the PalmTalk Forums tab) to see how the IPS and the Roy- al Botanic Gardens, Kew are partnering with a local village in Madagascar to help save this amazing palm. You can make a donation to the pro- ject and be a part of this unique partnership in conser- vation. Please donate today! One of the first photos posted on PalmTalk of the mysterious palm that came to be known as Tahina spectabilis and the little girl (at the time) for whom it was named, Anne-Tahina Metz. Volume 8.06 · July 2020 · Newsletter of the International Palm Society | Editor: Andy Hurwitz [email protected] Virtual hiking on Reunion Island, lowlands editionby Andy Hurwitz This article continues the “virtual hike” and tour of Reunion’s palms that began in last month’s News- letter. This month, we explore the palms and landscapes of the low to middle elevations. We begin by traveling to the windward eastern coast of the island, just south of Piton-Sainte-Rose, to ramble in Anse des Cascades. This idyllic cove is situated among groves of palm trees. The park is cool and shady. -

2951 – 3000 Trinidad to British Guiana
2951 – 3000 Trinidad to British Guiana [Inside cover] Book 9 Acanthophoenix 2956 Lecythis 2963 Acrocomia 2961 Licuala 2978 Ananas 2993 Livistona 2982 Archontophoenix 2983 Lodoicea 2985 Areca 2953 2953 Mauritia 2984 “ 2954 Mokka-mokka 2997 Astrocaryum 2957 Nipa 2981 “ 2986 Pachira 2976 “ 2987 “ 3000 Asystasia 2964 Passiflora 2952 Bauhinia 2960 “ 2995 Borassus 2979 Peltogyne 2970 Bromelia 2996 Pithecolobium 2965 Caryocar 2999 Randia 2994 Citrus 2998 Samanea 2966 Copernicia 2977 Undetermined 2967 Crotolaria 2973 “ In ink:]Ochna 2971 “ 2974 “ 2972 Desmoncus 2951 “ 2989 Diospyrus 2968 “ 2990 Euterpe 2955 “ 2991 Gmelina 2969 “ 2992 Hyphaene 2980 Zingerberaciae 2958 Ixora 2975 TILLANDSIA 2996 Jacaranda 2962 2951 Demoncus minor? See Harold Loomis’ notes and photo of inflorescence. #54 Villainously spacing & hooked climbing palm reminding one of those terrible Rattan palms of the Orient. When in fruit its bunches of deep scarlet fruits are attractive. A denizen of the deep shade forest and requiring moisture. From my experience in Coconut Grove with this genus I judge it wants half shade. Collected in Arena Forest Reserve Trinidad. 2/4/32 [In pencil] Loomis Photo 220 See D. F. Photo 18454-5 2952 P. H. Dorsett [In ink] “rubra?” Passiflora Sp Wild species of passion vine collected in the outskirts of the town of Eleuthera Bluff Island of Eleuthera Bahamas. Attractive looking species useful [Break in text, in ink] 1 single plant in pot Dec. 14/32. C. F. [Continuation of text] for breeding purposes. 1-7-32 (I’d like a few seeds or plants for my passiflora collection in Coconut Grove) Passiflora sp. Wild species growing in pothole in rocky soil of Eleuthera Bluff a colored town on Eleuthera Island. -

V20n3p119-120
t9761 PALM BRIEFS 119 PALM BRIEFS and again in January, 1970, aI the same or different localities within the same A NomenclqlurqlNole on district. The collector also observed Hyophorbe female and male inflorescences on the A monographic study of the genus same group of plants at dif{erent times. Hyophorbe is in preparation,but one o{ From the detailed description, the palm the conclusionsrequires advance publi- appears to be monocarpic, a character- cation in order to provide a name that istic of most of the caryotoid group of can be used in Hortus Third, and,in an- palms"(Moore, 1973). In monocarpic other publication. It has become clear habit, inflorescences develop basipetally that the genus Mascarena is not ade- from a terminal in{lorescence which is quately separated fuom Hyophorbe and generally a female, followed by axillary that the palm commonly cultivated as male inflorescences. This monocarpic Mascarena lagenicaulis must be trans- habit is seen in all three genera {erred to the older genus. Study has (Arenga, Caryota, Wallichia) of the shown that MascarenareuaughaniiL. H. caryotoid group. Arenga Labill. has Bailey is not different [rom M. lageni- imparipinnate or undivided leaves,often caulis, and in combining the two I am aggregate inflorescences, distinct sepals taking up the epithet that is descriptive and petals in staminate flowers, numer- and not likely to be confused with Hyo- ous stamens, trilocular ovary with 2-3 phorbe uaughanii. fertile locules, and homogeneousendo- The five species are: sperm. Caryota L. has bipinnate leaves, solitary inflorescences,reduction of fer- Hyophorbe amaricaulis Martius tile locules to I-2, and development of Hyophorbe indica Gaertner J. -

WRA Species Report
Family: Arecaceae Taxon: Hyophorbe verschaffeltii Synonym: Mascarena vershaffeltii L. H. Bailey Common Name: Spindle palm Palmiste Marron Questionaire : current 20090513 Assessor: Chuck Chimera Designation: L Status: Assessor Approved Data Entry Person: Chuck Chimera WRA Score -5 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- High substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 n 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 y 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see n Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see n Appendix 2) 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic y=1, n=0 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals y=1, n=-1 n 405 Toxic to animals y=1, n=0 n -

Monocotyledons and Gymnosperms of Puerto Rico and the Virgin Islands
SMITHSONIAN INSTITUTION Contributions from the United States National Herbarium Volume 52: 1-415 Monocotyledons and Gymnosperms of Puerto Rico and the Virgin Islands Editors Pedro Acevedo-Rodríguez and Mark T. Strong Department of Botany National Museum of Natural History Washington, DC 2005 ABSTRACT Acevedo-Rodríguez, Pedro and Mark T. Strong. Monocots and Gymnosperms of Puerto Rico and the Virgin Islands. Contributions from the United States National Herbarium, volume 52: 415 pages (including 65 figures). The present treatment constitutes an updated revision for the monocotyledon and gymnosperm flora (excluding Orchidaceae and Poaceae) for the biogeographical region of Puerto Rico (including all islets and islands) and the Virgin Islands. With this contribution, we fill the last major gap in the flora of this region, since the dicotyledons have been previously revised. This volume recognizes 33 families, 118 genera, and 349 species of Monocots (excluding the Orchidaceae and Poaceae) and three families, three genera, and six species of gymnosperms. The Poaceae with an estimated 89 genera and 265 species, will be published in a separate volume at a later date. When Ackerman’s (1995) treatment of orchids (65 genera and 145 species) and the Poaceae are added to our account of monocots, the new total rises to 35 families, 272 genera and 759 species. The differences in number from Britton’s and Wilson’s (1926) treatment is attributed to changes in families, generic and species concepts, recent introductions, naturalization of introduced species and cultivars, exclusion of cultivated plants, misdeterminations, and discoveries of new taxa or new distributional records during the last seven decades. -

Ornamental Garden Plants of the Guianas, Part 3
; Fig. 170. Solandra longiflora (Solanaceae). 7. Solanum Linnaeus Annual or perennial, armed or unarmed herbs, shrubs, vines or trees. Leaves alternate, simple or compound, sessile or petiolate. Inflorescence an axillary, extra-axillary or terminal raceme, cyme, corymb or panicle. Flowers regular, or sometimes irregular; calyx (4-) 5 (-10)- toothed; corolla rotate, 5 (-6)-lobed. Stamens 5, exserted; anthers united over the style, dehiscing by 2 apical pores. Fruit a 2-celled berry; seeds numerous, reniform. Key to Species 1. Trees or shrubs; stems armed with spines; leaves simple or lobed, not pinnately compound; inflorescence a raceme 1. S. macranthum 1. Vines; stems unarmed; leaves pinnately compound; inflorescence a panicle 2. S. seaforthianum 1. Solanum macranthum Dunal, Solanorum Generumque Affinium Synopsis 43 (1816). AARDAPPELBOOM (Surinam); POTATO TREE. Shrub or tree to 9 m; stems and leaves spiny, pubescent. Leaves simple, toothed or up to 10-lobed, to 40 cm. Inflorescence a 7- to 12-flowered raceme. Corolla 5- or 6-lobed, bluish-purple, to 6.3 cm wide. Range: Brazil. Grown as an ornamental in Surinam (Ostendorf, 1962). 2. Solanum seaforthianum Andrews, Botanists Repository 8(104): t.504 (1808). POTATO CREEPER. Vine to 6 m, with petiole-tendrils; stems and leaves unarmed, glabrous. Leaves pinnately compound with 3-9 leaflets, to 20 cm. Inflorescence a many- flowered panicle. Corolla 5-lobed, blue, purple or pinkish, to 5 cm wide. Range:South America. Grown as an ornamental in Surinam (Ostendorf, 1962). Sterculiaceae Monoecious, dioecious or polygamous trees and shrubs. Leaves alternate, simple to palmately compound, petiolate. Inflorescence an axillary panicle, raceme, cyme or thyrse. -

ISSN: 0975-8585 March – April 2016 RJPBCS 7(2) Page No
ISSN: 0975-8585 Research Journal of Pharmaceutical, Biological and Chemical Sciences Hyophorbe verschaffeltii DNA Profiling, Chemical Composition of the Lipophilic Fraction, Antimicrobial, Anti-Inflammatory and Cytotoxic Activities. Shaza H Aly1, Mohamed R Elgindi3,4, Abd El-Nassar B Singab2, and Ibrahim I Mahmoud4,5. 1Department of Pharmacognosy, Faculty of Pharmacy, Badr University in Cairo, Cairo, Egypt 2Department of Pharmacognosy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt 3Department of Pharmacognosy, Faculty of Pharmacy, Egyptian Russian University,Cairo, Egypt. 4Department of Pharmacognosy, Faculty of Pharmacy, Helwan University, Cairo, Egypt. 5Department of Pharmacognosy, Faculty of Pharmacy, Al Ahram Canadian University, Cairo, Egypt. ABSTRACT To authenticate Hyophorbe verschaffeltii with investigation of lipoidal matters and biological activities. DNA profiling was carried out by random amplified polymorphic DNA-PCR. Petroleum ether extract was investigated for lipoidal matters using GC-MS. Anti-inflammatory activity was assayed in vivo by Carrageenan-induced rat hind paw edema technique, Antimicrobial screening was done by a standard agar well diffusion method and cytotoxicity assay was measured against MCF-7 cells using the MTT Cell Viability Assay. The ten primers used for RAPD-PCR analysis produced totally 73 amplified DNA fragments and primer OPA-12 was the best sequence for dominating Hyophorbe verschaffeltii producing the highest hits (10).The results of the lipoidal matter investigation revealed the presence of squalene (15.40%), phytol (4.10%), myristic acid (13.20%), undecanoic acid (11.87%) and pentadecanoic acid (11.24%). Aqueous methanol extract exhibited cytotoxicity activity at IC 50(323.6 μg/ml) against MCF-7 cells, anti-inflammatory activity and antimicrobial activity against Bacillus subtillus, Escherichia Coli, Pseudomonas Aeruginosa and Candida albicans. -

Atoll Research Bulletin No. 503 the Vascular Plants Of
ATOLL RESEARCH BULLETIN NO. 503 THE VASCULAR PLANTS OF MAJURO ATOLL, REPUBLIC OF THE MARSHALL ISLANDS BY NANCY VANDER VELDE ISSUED BY NATIONAL MUSEUM OF NATURAL HISTORY SMITHSONIAN INSTITUTION WASHINGTON, D.C., U.S.A. AUGUST 2003 Uliga Figure 1. Majuro Atoll THE VASCULAR PLANTS OF MAJURO ATOLL, REPUBLIC OF THE MARSHALL ISLANDS ABSTRACT Majuro Atoll has been a center of activity for the Marshall Islands since 1944 and is now the major population center and port of entry for the country. Previous to the accompanying study, no thorough documentation has been made of the vascular plants of Majuro Atoll. There were only reports that were either part of much larger discussions on the entire Micronesian region or the Marshall Islands as a whole, and were of a very limited scope. Previous reports by Fosberg, Sachet & Oliver (1979, 1982, 1987) presented only 115 vascular plants on Majuro Atoll. In this study, 563 vascular plants have been recorded on Majuro. INTRODUCTION The accompanying report presents a complete flora of Majuro Atoll, which has never been done before. It includes a listing of all species, notation as to origin (i.e. indigenous, aboriginal introduction, recent introduction), as well as the original range of each. The major synonyms are also listed. For almost all, English common names are presented. Marshallese names are given, where these were found, and spelled according to the current spelling system, aside from limitations in diacritic markings. A brief notation of location is given for many of the species. The entire list of 563 plants is provided to give the people a means of gaining a better understanding of the nature of the plants of Majuro Atoll. -

A Synopsis of the Pre-Human Avifauna of the Mascarene Islands
– 195 – Paleornithological Research 2013 Proceed. 8th Inter nat. Meeting Society of Avian Paleontology and Evolution Ursula B. Göhlich & Andreas Kroh (Eds) A synopsis of the pre-human avifauna of the Mascarene Islands JULIAN P. HUME Bird Group, Department of Life Sciences, The Natural History Museum, Tring, UK Abstract — The isolated Mascarene Islands of Mauritius, Réunion and Rodrigues are situated in the south- western Indian Ocean. All are volcanic in origin and have never been connected to each other or any other land mass. Despite their comparatively close proximity to each other, each island differs topographically and the islands have generally distinct avifaunas. The Mascarenes remained pristine until recently, resulting in some documentation of their ecology being made before they rapidly suffered severe degradation by humans. The first major fossil discoveries were made in 1865 on Mauritius and on Rodrigues and in the late 20th century on Réunion. However, for both Mauritius and Rodrigues, the documented fossil record initially was biased toward larger, non-passerine bird species, especially the dodo Raphus cucullatus and solitaire Pezophaps solitaria. This paper provides a synopsis of the fossil Mascarene avifauna, which demonstrates that it was more diverse than previously realised. Therefore, as the islands have suffered severe anthropogenic changes and the fossil record is far from complete, any conclusions based on present avian biogeography must be viewed with caution. Key words: Mauritius, Réunion, Rodrigues, ecological history, biogeography, extinction Introduction ily described or illustrated in ships’ logs and journals, which became the source material for The Mascarene Islands of Mauritius, Réunion popular articles and books and, along with col- and Rodrigues are situated in the south-western lected specimens, enabled monographs such as Indian Ocean (Fig. -

19 Annual Meeting of the Society for Conservation Biology BOOK of ABSTRACTS
19th Annual Meeting of the Society for Conservation Biology BOOK OF ABSTRACTS Universidade de Brasília Universidade de Brasília Brasília, DF, Brazil 15th -19th July 2005 Universidade de Brasília, Brazil, July 2005 Local Organizing Committees EXECUTIVE COMMITTEE SPECIAL EVENTS COMMITTEE Miguel Ângelo Marini, Chair (OPENING, ALUMNI/250TH/BANQUET) Zoology Department, Universidade de Brasília, Brazil Danielle Cavagnolle Mota (Brazil), Chair Jader Soares Marinho Filho Regina Macedo Zoology Department, Universidade de Brasília, Brazil Fiona Nagle (Topic Area Networking Lunch) Regina Helena Ferraz Macedo Camilla Bastianon (Brazil) Zoology Department, Universidade de Brasília, Brazil John Du Vall Hay Ecology Department, Universidade de Brasília, Brazil WEB SITE COMMITTEE Isabella Gontijo de Sá (Brazil) Delchi Bruce Glória PLENARY, SYMPOSIUM, WORKSHOP AND Rafael Cerqueira ORGANIZED DISCUSSION COMMITTEE Miguel Marini, Chair Jader Marinho PROGRAM LOGISTICS COMMITTEE Regina Macedo Paulo César Motta (Brazil), Chair John Hay Danielle Cavagnolle Mota Jon Paul Rodriguez Isabella de Sá Instituto Venezolano de Investigaciones Científicas (IVIC), Venezuela Javier Simonetti PROGRAM AND ABSTRACTS COMMITTEE Departamento de Ciencias Ecológicas, Facultad de Cien- cias, Universidad de Chile, Chile Reginaldo Constantino (Brazil), Chair Gustavo Fonseca Débora Goedert Conservation International, USA and Universidade Federal de Minas Gerais, Brazil Eleanor Sterling SHORT-COURSES COMMITTEE American Museum of Natural History, USA Guarino Rinaldi Colli (Brazil), Chair