CHEE 221 Tutorial: Saturation and Steam Tables What Do They Really
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advanced Organic Vapor Cycles for Improving Thermal Conversion Efficiency in Renewable Energy Systems by Tony Ho a Dissertation
Advanced Organic Vapor Cycles for Improving Thermal Conversion Efficiency in Renewable Energy Systems By Tony Ho A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Engineering – Mechanical Engineering in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Ralph Greif, Co-Chair Professor Samuel S. Mao, Co-Chair Professor Van P. Carey Professor Per F. Peterson Spring 2012 Abstract Advanced Organic Vapor Cycles for Improving Thermal Conversion Efficiency in Renewable Energy Systems by Tony Ho Doctor of Philosophy in Mechanical Engineering University of California, Berkeley Professor Ralph Greif, Co-Chair Professor Samuel S. Mao, Co-Chair The Organic Flash Cycle (OFC) is proposed as a vapor power cycle that could potentially increase power generation and improve the utilization efficiency of renewable energy and waste heat recovery systems. A brief review of current advanced vapor power cycles including the Organic Rankine Cycle (ORC), the zeotropic Rankine cycle, the Kalina cycle, the transcritical cycle, and the trilateral flash cycle is presented. The premise and motivation for the OFC concept is that essentially by improving temperature matching to the energy reservoir stream during heat addition to the power cycle, less irreversibilities are generated and more power can be produced from a given finite thermal energy reservoir. In this study, modern equations of state explicit in Helmholtz energy such as the BACKONE equations, multi-parameter Span- Wagner equations, and the equations compiled in NIST REFPROP 8.0 were used to accurately determine thermodynamic property data for the working fluids considered. Though these equations of state tend to be significantly more complex than cubic equations both in form and computational schemes, modern Helmholtz equations provide much higher accuracy in the high pressure regions, liquid regions, and two-phase regions and also can be extended to accurately describe complex polar fluids. -

Structural Transformation in Supercooled Water Controls the Crystallization Rate of Ice
Structural transformation in supercooled water controls the crystallization rate of ice. Emily B. Moore and Valeria Molinero* Department of Chemistry, University of Utah, Salt Lake City, UT 84112-0580, USA. Contact Information for the Corresponding Author: VALERIA MOLINERO Department of Chemistry, University of Utah 315 South 1400 East, Rm 2020 Salt Lake City, UT 84112-0850 Phone: (801) 585-9618; fax (801)-581-4353 Email: [email protected] 1 One of water’s unsolved puzzles is the question of what determines the lowest temperature to which it can be cooled before freezing to ice. The supercooled liquid has been probed experimentally to near the homogeneous nucleation temperature T H≈232 K, yet the mechanism of ice crystallization—including the size and structure of critical nuclei—has not yet been resolved. The heat capacity and compressibility of liquid water anomalously increase upon moving into the supercooled region according to a power law that would diverge at T s≈225 K,1,2 so there may be a link between water’s thermodynamic anomalies and the crystallization rate of ice. But probing this link is challenging because fast crystallization prevents experimental studies of the liquid below T H. And while atomistic studies have captured water crystallization3, the computational costs involved have so far prevented an assessment of the rates and mechanism involved. Here we report coarse-grained molecular simulations with the mW water model4 in the supercooled regime around T H, which reveal that a sharp increase in the fraction of four-coordinated molecules in supercooled liquid water explains its anomalous thermodynamics and also controls the rate and mechanism of ice formation. -

TABLE A-2 Properties of Saturated Water (Liquid–Vapor): Temperature Table Specific Volume Internal Energy Enthalpy Entropy # M3/Kg Kj/Kg Kj/Kg Kj/Kg K Sat
720 Tables in SI Units TABLE A-2 Properties of Saturated Water (Liquid–Vapor): Temperature Table Specific Volume Internal Energy Enthalpy Entropy # m3/kg kJ/kg kJ/kg kJ/kg K Sat. Sat. Sat. Sat. Sat. Sat. Sat. Sat. Temp. Press. Liquid Vapor Liquid Vapor Liquid Evap. Vapor Liquid Vapor Temp. Њ v ϫ 3 v Њ C bar f 10 g uf ug hf hfg hg sf sg C O 2 .01 0.00611 1.0002 206.136 0.00 2375.3 0.01 2501.3 2501.4 0.0000 9.1562 .01 H 4 0.00813 1.0001 157.232 16.77 2380.9 16.78 2491.9 2508.7 0.0610 9.0514 4 5 0.00872 1.0001 147.120 20.97 2382.3 20.98 2489.6 2510.6 0.0761 9.0257 5 6 0.00935 1.0001 137.734 25.19 2383.6 25.20 2487.2 2512.4 0.0912 9.0003 6 8 0.01072 1.0002 120.917 33.59 2386.4 33.60 2482.5 2516.1 0.1212 8.9501 8 10 0.01228 1.0004 106.379 42.00 2389.2 42.01 2477.7 2519.8 0.1510 8.9008 10 11 0.01312 1.0004 99.857 46.20 2390.5 46.20 2475.4 2521.6 0.1658 8.8765 11 12 0.01402 1.0005 93.784 50.41 2391.9 50.41 2473.0 2523.4 0.1806 8.8524 12 13 0.01497 1.0007 88.124 54.60 2393.3 54.60 2470.7 2525.3 0.1953 8.8285 13 14 0.01598 1.0008 82.848 58.79 2394.7 58.80 2468.3 2527.1 0.2099 8.8048 14 15 0.01705 1.0009 77.926 62.99 2396.1 62.99 2465.9 2528.9 0.2245 8.7814 15 16 0.01818 1.0011 73.333 67.18 2397.4 67.19 2463.6 2530.8 0.2390 8.7582 16 17 0.01938 1.0012 69.044 71.38 2398.8 71.38 2461.2 2532.6 0.2535 8.7351 17 18 0.02064 1.0014 65.038 75.57 2400.2 75.58 2458.8 2534.4 0.2679 8.7123 18 19 0.02198 1.0016 61.293 79.76 2401.6 79.77 2456.5 2536.2 0.2823 8.6897 19 20 0.02339 1.0018 57.791 83.95 2402.9 83.96 2454.1 2538.1 0.2966 8.6672 20 21 0.02487 1.0020 -

Spinodal Lines and Equations of State: a Review
10 l Nuclear Engineering and Design 95 (1986) 297-314 297 North-Holland, Amsterdam SPINODAL LINES AND EQUATIONS OF STATE: A REVIEW John H. LIENHARD, N, SHAMSUNDAR and PaulO, BINEY * Heat Transfer/ Phase-Change Laboratory, Mechanical Engineering Department, University of Houston, Houston, TX 77004, USA The importance of knowing superheated liquid properties, and of locating the liquid spinodal line, is discussed, The measurement and prediction of the spinodal line, and the limits of isentropic pressure undershoot, are reviewed, Means are presented for formulating equations of state and fundamental equations to predict superheated liquid properties and spinodal limits, It is shown how the temperature dependence of surface tension can be used to verify p - v - T equations of state, or how this dependence can be predicted if the equation of state is known. 1. Scope methods for making simplified predictions of property information, which can be applied to the full range of Today's technology, with its emphasis on miniaturiz fluids - water, mercury, nitrogen, etc. [3-5]; and predic ing and intensifying thennal processes, steadily de tions of the depressurizations that might occur in ther mands higher heat fluxes and poses greater dangers of mohydraulic accidents. (See e.g. refs. [6,7].) sending liquids beyond their boiling points into the metastable, or superheated, state. This state poses the threat of serious thermohydraulic explosions. Yet we 2. The spinodal limit of liquid superheat know little about its thermal properties, and cannot predict process behavior after a liquid becomes super heated. Some of the practical situations that require a 2.1. The role of the equation of state in defining the spinodal line knowledge the limits of liquid superheat, and the physi cal properties of superheated liquids, include: - Thennohydraulic explosions as might occur in nuclear Fig. -

Subcritical Water Extraction
Chapter 17 Subcritical Water Extraction A. Haghighi Asl and M. Khajenoori Additional information is available at the end of the chapter http://dx.doi.org/10.5772/54993 1. Introduction Extraction always involves a chemical mass transfer from one phase to another. The principles of extraction are used to advantage in everyday life, for example in making juices, coffee and others. To reduce the use of organic solvent and improve the extraction methods of constituents of plant materials, new methods such as microwave assisted extraction (MAE), supercritical fluid extraction (SFE), accelerated solvent extraction (ASE) or pressurized liquid extraction (PLE) and subcritical water extraction (SWE), also called superheated water extraction or pressurized hot water extraction (PHWE), have been introduced [1-3]. SWE is a new and powerful technique at temperatures between 100 and 374oC and pressure high enough to maintain the liquid state (Fig.1) [4]. Unique properties of water are namely its disproportionately high boiling point for its mass, a high dielectric constant and high polarity [4]. As the temperature rises, there is a marked and systematic decrease in permittivity, an increase in the diffusion rate and a decrease in the viscosity and surface tension. In conse‐ quence, more polar target materials with high solubility in water at ambient conditions are extracted most efficiently at lower temperatures, whereas moderately polar and non-polar targets require a less polar medium induced by elevated temperature [5]. Based on the research works published in the recent years, it has been shown that the SWE is cleaner, faster and cheaper than the conventional extraction methods. -
Thermodynamic Data
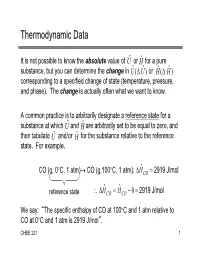
Thermodynamic Data It is not possible to know the absolute value of U ˆ or H ˆ for a pure substance, but you can determine the change in U ˆ ( U ˆ ) or Hˆ ( Hˆ ) corresponding to a specified change of state (temperature, pressure, and phase). The change is actually often what we want to know. A common practice is to arbitrarily designate a reference state for a substance at which U ˆ and H ˆ are arbitrarily set to be equal to zero, and then tabulate U ˆ and/or H ˆ for the substance relative to the reference state. For example, ˆ CO (g, 0C, 1 atm) CO (g,100C, 1 atm): HCO 2919 J/mol ˆ ˆ reference state HCO HCO 0 2919 J/mol We say: “The specific enthalpy of CO at 100C and 1 atm relative to CO at 0C and 1 atm is 2919 J/mol”. CHEE 221 1 Reference States and State Properties Most (all?) enthalpy tables report the reference states (T, P and State) on which the values of H ˆ are based; however, it is not necessary to know the reference state to calculate H (change in enthalpy) for the transition from one state to another. ˆ ˆ –H from state 1 to state 2 equals H 2 H 1 regardless of the reference state upon which ˆ and ˆ were based H1 H 2 – Caution: If you use different tables, you must make sure they have the same reference state This result is a consequence of the fact that H ˆ (and U ˆ ) are state properties, that is, their values depend only on the state of the species (temperature, pressure, state) and not on how the species reached its state. -

Source Term Models for Superheated Releases of Hazardous Materials
Source Term Models for Superheated Releases of Hazardous Materials Thesis submitted for the Degree of Doctor of Philosophy of the University of Wales Cardiff by Vincent Martin Cleary BEng (Hons) September 2008 UMI Number: U585126 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI U585126 Published by ProQuest LLC 2013. Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 Chapter 1 Introduction Abstract Source terms models for superheated releases of hazardous liquefied chemicals such as LPG have been developed, governing both upstream and downstream conditions. Water was utilised as the model fluid, not least for reasons of safety, but also for its ability to be stored at conditions that ensure it is superheated on release to atmosphere. Several studies have found that at low superheat jet break-up is analogous to mechanical break-up under sub-cooled conditions. Hence, a non-dimensionalised SMD correlation for sub-cooled liquid jets in the atomisation regime has been developed, based on data measured using a Phase Doppler Anemometry (PDA) system, for a broad range of initial conditions. Droplet SMD has been found to correlate with the nozzle aspect ratio and two non- dimensionalised groups i.e. -

Backpressure Steam Power Generation in District Energy and CHP - Energy Efficiency Considerations
Backpressure Steam Power Generation in District Energy and CHP - Energy Efficiency Considerations A 1st Law, 2nd Law and Economic Analysis of the Practical Steam Engine in a District Energy/CHP Application Joshua A. Tolbert, Ph.D. Practical Steam Energy Efficiency Engineering – How is efficiency calculated? • What is the Status Quo? – Engineers currently focus on limiting energy losses as the primary point of focus for district energy and CHP systems – Only local energy losses are typically considered causing energy efficiency opportunities to be wasted • Why? – Conventional wisdom tells us that minimizing local energy losses is ultimate goal • How should we change? – Could a different approach improve global efficiency? Motivation Common Questions: 1. How can installing an imperfect device in parallel to an isenthalpic pressure reducing valve (PRV) improve efficiency? 2. My analysis shows an incremental fuel cost with PRV parallel, how can this be more efficient? Analysis Overview • Consider a Practical Steam Engine (PSE) in a Pressure Reducing Valve (PRV) parallel district energy application • PSE operation is consistent with CHP application • Consider 1st Law, 2nd Law and economic analysis for energy efficiency • Draw conclusions Backpressure Application – Case Study Enwave Seattle - District Energy / CHP Application Assumptions Equipment • 1.5 MW (5.118 MMBtu/hr) • Practical Steam Engine (PSE) heating load is considered o Isentropic efficiency of 80% • Condensate exiting load: o Mechanical efficiency of 80% Saturate liquid at 20 psig o Generator efficiency of 95% • No heat losses in piping or • Isenthalpic PRV equipment* • 150 psig saturated steam boiler • 80% boiler and feedwater pump efficiency *Incorporating actual heat losses does not significantly affect results of analysis. -

Superheated Steam in Locomotive Service
I LL INO I S UNIVERSITY OF ILLINOIS AT URBANA-CHAMPAIGN PRODUCTION NOTE University of Illinois at Urbana-Champaign Library Large-scale Digitization Project, 2007. 0 ~0' / ~ ( ~ 1~CC~& '~f~LW "0~ "N ~ ~- ~N~TV N Q SC 2"' C' ''~~C'~ C" 4 AA.A..~ Ay ~4.A A A'-' 'A ' ~ ' ,~A~A 1 A' 'A": "I ~' ~A 9 ~AA >' 0 -A.'-- ~ A" Z 'A A' * A -A "* K~ A. A' ~. 'A A, ,~, 'U",' 4 *, A' '~'" 'A * JA A' 'A' A A K 'A 'A / A A A' 'A vas,^esta>bflibea~ ~ by ^f'A A' A ,rt~7B A 'A on, investigations~ve~Wg~on.~. A' ~ 'i* *t~.,to studysti~dy~ prob1~m~problems A sS ~4 IOAWeto eadA manu-m&i~n- 'be industrial interests 'A' of theb8tat;' -'$a te' The control of tho Engineering ExperintSerment• 'titionSl i•is is'vested vested 'A UNIVERSITY OF ILLINOIS ENGINEERING EXPERIMENT STATION BULLETIN No. 57 APRIL 1912 SUPERHEATED STEAM IN LOCOMOTIVE SERVICE (A REVIEW OF PUBLICATION NO. 127 OF THE CARNEGIE INSTITUTION OF WASHINGTON) BY W. F. M. GOSS DEAN OF THE COLLEGE OF ENGINEERING DIRECTOR OF THE ENGINEERING EXPERIMENT STATION DIRECTOR OF THE SCHOOL OF RAILWAY ENGINEERING AND ADMINISTRATION CONTENTS PAGE I. Introduction-A Summary of Conclusions .......... 3 II. Foreign Practice in the Use of Superheated Steam in Locomotive Service.............. ......... 5 III. Tests to Determine the Value of Superheating in Lo- comotive Service................ .. .. .... 14 IV. Performance of Boiler and Superheater.. ........... 20 V. Performance of the Engine and of the Locomotive as a W hole... ............ .. .. ........ 35 VI. Economy Resulting from the Use of Superheated Steam ........... -

The Relationship Between Liquid, Supercooled and Glassy Water
review article The relationship between liquid, supercooled and glassy water Osamu Mishima & H. Eugene Stanley 8 ........................................................................................................................................................................................................................................................ That water can exist in two distinct `glassy' formsÐlow- and high-density amorphous iceÐmay provide the key to understanding some of the puzzling characteristics of cold and supercooled water, of which the glassy solids are more- viscous counterparts. Recent experimental and theoretical studies of both liquid and glassy water are now starting to offer the prospect of a coherent picture of the unusual properties of this ubiquitous substance. At least one `mysterious' property of liquid water seems to have been unreachable temperature Ts < 2 45 8C (refs 2, 5), at which point recognized 300 years ago1: whereas most liquids contract as tem- the entire concept of a `liquid state' becomes dif®cult to sustain. perature decreases, liquid water begins to expand when its tem- Water is a liquid, but glassy waterÐalso called amorphous iceÐ perature drops below 4 8C. A kitchen experiment demonstrates that can exist when the temperature drops below the glass transition the bottom layer of a glass of unstirred iced water remains at 4 8C temperature Tg (about 130 K at 1 bar). Although glassy water is a while colder layers `¯oat' on top; the temperature at the bottom rises solid, its structure exhibits a disordered liquid-like arrangement. only after all the ice has melted. Low-density amorphous ice (LDA) has been known to exist for The mysterious properties of liquid water become more pro- 60 years (ref. 6), and a second kind of amorphous ice, high-density nounced in the supercooled region below 0 8C (refs 2±4; Fig. -

Rapid Evaporation at the Superheat Limit of Methanol, Ethanol, Butanol and N-Heptane on Platinum films Supported by Low-Stress Sin Membranes ⇑ Eric J
International Journal of Heat and Mass Transfer 101 (2016) 707–718 Contents lists available at ScienceDirect International Journal of Heat and Mass Transfer journal homepage: www.elsevier.com/locate/ijhmt Rapid evaporation at the superheat limit of methanol, ethanol, butanol and n-heptane on platinum films supported by low-stress SiN membranes ⇑ Eric J. Ching a,1, C. Thomas Avedisian a, , Richard C. Cavicchi b, Do Hyun Chung a,2, Kyupaeck J. Rah a,3, Michael J. Carrier b a Sibley School of Mechanical and Aerospace Engineering, Cornell University, Ithaca, NY 14853, USA b Biomolecular Measurement Division, National Institute of Standards and Technology, Gaithersburg, MD 20899, USA article info abstract Article history: The bubble nucleation temperatures of several organic liquids (methanol, ethanol, butanol, n-heptane) on Received 30 December 2015 stress-minimized platinum (Pt) films supported by SiN membranes is examined by pulse-heating the Received in revised form 3 April 2016 membranes for times ranging from 1 lsto10ls. The results show that the nucleation temperatures Accepted 3 April 2016 increase as the heating rates of the Pt films increase. Measured nucleation temperatures approach predicted superheat limits for the smallest pulse times which correspond to heating rates over 108 K/s, while nucleation temperatures are significantly lower for the longest pulse times. The microheater mem- Keywords: branes were found to be robust for millions of pulse cycles, which suggests their potential in applications Homogeneous nucleation for moving fluids on the microscale and for more fundamental studies of phase transitions of metastable Boiling Superheat limit liquids. Bubble nucleation Ó 2016 Published by Elsevier Ltd. -

Subcritical Water As a Tunable Solvent for Particle Engineering
SUBCRITICAL WATER AS A TUNABLE SOLVENT FOR PARTICLE ENGINEERING By Adam Carr, B.E. (Chemical Engineering) A thesis submitted to the School of Chemical Engineering in partial fulfillment of the requirements of the degree: Doctor of Philosophy (PhD) The University of New South Wales May 2010 ABSTRACT In the research described in this thesis, an environmentally friendly technique for engineering the morphology of particles was developed. Active pharmaceutical ingredients (APIs) were used as model compounds to explore the potential of the new particle engineering technology. APIs were chosen because tuning the size and shape of drug particles can have pharmacokinetic benefits. The new technology used subcritical water (SBCW) as a solvent to dissolve APIs. By rapidly cooling a SBCW-API solution, the dissolved drug was rapidly precipitated, often with a narrow particle size distribution. Furthermore, it was shown that the morphology of the precipitated particles can be changed by altering several process variables. In order to develop a new precipitation technology, fundamental solubility data were required. Solubility data were collected for the model APIs; budesonide, griseofulvin, naproxen and pyrimethamine in SBCW from 100°C to 200°C. To ensure that the solubility results were reliable, data were also collected for anthracene, which were compared to published SBCW solubility data. The solubility of budesonide in SBCW was low. Organic solvents were added to the SBCW-budesonide solution to increase the solubility of the API. A model that correlated the solubility of the solute in SBCW with the dielectric constant of the solvent was developed. Model outputs were within 5% of the experimental solubility values.