Studies of the Physiology of Lymphatic Vessel by Microcirculation Methods*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heart Vein Artery
1 PRE-LAB EXERCISES Open the Atlas app. From the Views menu, go to System Views and scroll down to Circulatory System Views. You are responsible for the identification of all bold terms. A. Circulatory System Overview In the Circulatory System Views section, select View 1. Circulatory System. The skeletal system is included in this view. Note that blood vessels travel throughout the entire body. Heart Artery Vein 2 Brachiocephalic trunk Pulmonary circulation Pericardium 1. Where would you find the blood vessels with the largest diameter? 2. Select a few vessels in the leg and read their names. The large blue-colored vessels are _______________________________ and the large red-colored vessels are_______________________________. 3. In the system tray on the left side of the screen, deselect the skeletal system icon to remove the skeletal system structures from the view. The largest arteries and veins are all connected to the _______________________________. 4. Select the heart to highlight the pericardium. Use the Hide button in the content box to hide the pericardium from the view and observe the heart muscle and the vasculature of the heart. 3 a. What is the largest artery that supplies the heart? b. What are the two large, blue-colored veins that enter the right side of the heart? c. What is the large, red-colored artery that exits from the top of the heart? 5. Select any of the purple-colored branching vessels inside the rib cage and use the arrow in the content box to find and choose Pulmonary circulation from the hierarchy list. This will highlight the circulatory route that takes deoxygenated blood to the lungs and returns oxygenated blood back to the heart. -

29 Assessing the Cardiovascular and Lymphatic Systems
29 Assessing the Cardiovascular and Lymphatic Systems LEARNING OUTCOMES 1. Describe the anatomy, physiology, and functions of the 5. Explain techniques used to assess cardiovascular and cardiovascular and lymphatic systems. lymphatic structure and function. 2. Describe normal variations in cardiovascular assessment 6. Identify manifestations of impaired cardiovascular structure findings for the older adult. and functions. 3. Give examples of genetic disorders of the cardiovascular system. 4. Identify specific topics for consideration during a health history assessment interview of the patient with cardiovascu- lar or lymphatic disorders. CLINICAL COMPETENCIES 1. Complete a health history for patients having alterations in 3. Assess an ECG strip and identify normal rhythm and cardiac the structure and functions of the cardiovascular or lymphatic events and abnormal cardiac rhythm. systems. 4. Monitor the results of diagnostic tests and communicate 2. Conduct and document a physical assessment of cardiovas- abnormal findings within the interprofessional team. cular and lymphatic status. MAJOR CHAPTER CONCEPTS • Correct structure and function of the cardiovascular and • Manifestations of dysfunction, injury, and disorders affecting lymphatic systems are vital to the transport of oxygen and the cardiovascular and lymphatic systems may be detected carbon dioxide throughout the body and for the return of during a general health assessment as well as during focused excess tissue fluids back to the bloodstream. cardiovascular and lymphatic system -

Pelvic Anatomyanatomy
PelvicPelvic AnatomyAnatomy RobertRobert E.E. Gutman,Gutman, MDMD ObjectivesObjectives UnderstandUnderstand pelvicpelvic anatomyanatomy Organs and structures of the female pelvis Vascular Supply Neurologic supply Pelvic and retroperitoneal contents and spaces Bony structures Connective tissue (fascia, ligaments) Pelvic floor and abdominal musculature DescribeDescribe functionalfunctional anatomyanatomy andand relevantrelevant pathophysiologypathophysiology Pelvic support Urinary continence Fecal continence AbdominalAbdominal WallWall RectusRectus FasciaFascia LayersLayers WhatWhat areare thethe layerslayers ofof thethe rectusrectus fasciafascia AboveAbove thethe arcuatearcuate line?line? BelowBelow thethe arcuatearcuate line?line? MedianMedial umbilicalumbilical fold Lateralligaments umbilical & folds folds BonyBony AnatomyAnatomy andand LigamentsLigaments BonyBony PelvisPelvis TheThe bonybony pelvispelvis isis comprisedcomprised ofof 22 innominateinnominate bones,bones, thethe sacrum,sacrum, andand thethe coccyx.coccyx. WhatWhat 33 piecespieces fusefuse toto makemake thethe InnominateInnominate bone?bone? PubisPubis IschiumIschium IliumIlium ClinicalClinical PelvimetryPelvimetry WhichWhich measurementsmeasurements thatthat cancan bebe mademade onon exam?exam? InletInlet DiagonalDiagonal ConjugateConjugate MidplaneMidplane InterspinousInterspinous diameterdiameter OutletOutlet TransverseTransverse diameterdiameter ((intertuberousintertuberous)) andand APAP diameterdiameter ((symphysissymphysis toto coccyx)coccyx) -

Vascular Density and Distribution in Neocortex
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2019 Vascular density and distribution in neocortex Schmid, Franca ; Barrett, Matthew J P ; Jenny, Patrick ; Weber, Bruno Abstract: An amazingly wide range of complex behavior emerges from the cerebral cortex. Much of the information processing that leads to these behaviors is performed in neocortical circuits that span throughout the six layers of the cortex. Maintaining this circuit activity requires substantial quantities of oxygen and energy substrates, which are delivered by the complex yet well-organized and tightly-regulated vascular system. In this review, we provide a detailed characterization of the most relevant anatomical and functional features of the cortical vasculature. This includes a compilation of the available data on laminar variation of vascular density and the topological aspects of the microvascular system. We also review the spatio-temporal dynamics of cortical blood flow regulation and oxygenation, many aspects of which remain poorly understood. Finally, we discuss some of the important implications of vascular density, distribution, oxygenation and blood flow regulation for (laminar) fMRI. DOI: https://doi.org/10.1016/j.neuroimage.2017.06.046 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-146003 Journal Article Accepted Version The following work is licensed under a Creative Commons: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) License. Originally published at: Schmid, Franca; Barrett, Matthew J P; Jenny, Patrick; Weber, Bruno (2019). Vascular density and distribution in neocortex. -

Machine-Learning-Based Functional Microcirculation Analysis
The Thirty-Second Innovative Applications of Artificial Intelligence Conference (IAAI-20) Machine-Learning-Based Functional Microcirculation Analysis Ossama Mahmoud1, GH Janssen2,3, Mahmoud R. El-Sakka1 1 Department of Computer Sciences, Western University, London (ON), Canada 2 Department of Medical Biophysics, Western University, London (ON), Canada 3 Centre for Critical Illness Research, Lawson Health Research Institute, London (ON), Canada Abstract The functionality of these microvessels, i.e., the ability to Analysis of microcirculation is an important clinical and re- carry blood flow, has significant implications for organ search task. Functional analysis of the microcirculation al- function during disease progression and overall health. As lows researchers to understand how blood flowing in a tis- such, IVM is used in various medical domains to examine sues’ smallest vessels affects disease progression, organ the microcirculation and to understand disease processes function, and overall health. Current methods of manual analysis of microcirculation are tedious and time- and their effects on the microcirculation (Ellis 2005; consuming, limiting the quick turnover of results. There has Lawendy 2016; Yeh 2017). been limited research on automating functional analysis of The quality of a tissue’s microcirculation can be meas- microcirculation. As such, in this paper, we propose a two- ured through its vascular density. Usually, vascular density step machine-learning-based algorithm to functionally as- for a region of tissue is determined by taking the total sess microcirculation videos. The first step uses a modified vessel segmentation algorithm to extract the location of ves- number of flowing microvessels across a cross-section sel-like structures. While the second step uses a 3D-CNN to divided by the surface area of the region examined (Charl- assess whether the vessel-like structures contained flowing ton 2017). -
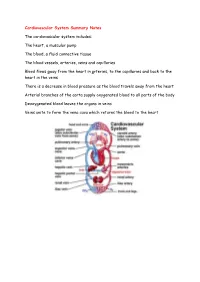
Cardiovascular System Summary Notes the Cardiovascular System
Cardiovascular System Summary Notes The cardiovascular system includes: The heart, a muscular pump The blood, a fluid connective tissue The blood vessels, arteries, veins and capillaries Blood flows away from the heart in arteries, to the capillaries and back to the heart in the veins There is a decrease in blood pressure as the blood travels away from the heart Arterial branches of the aorta supply oxygenated blood to all parts of the body Deoxygenated blood leaves the organs in veins Veins unite to form the vena cava which returns the blood to the heart Pulmonary System This is the route by which blood is circulated from the heart to the lungs and back to the heart again The pulmonary system is exceptional in that the pulmonary artery carries deoxygenated blood and the pulmonary vein carries oxygenated blood Hepatic Portal Vein There is another exception in the circulatory system – the hepatic portal vein Veins normally carry blood from an organ back to the heart The hepatic portal vein carries blood from the capillary bed of the intestine to the capillary bed of the liver As a result, the liver has three blood vessels associated with it Arteries and Veins The central cavity of a blood vessel is called the lumen The lumen is lined with a thin layer of cells called the endothelium The composition of the vessel wall surrounding the endothelium is different in arteries, veins and capillaries Arteries carry blood away from the heart Arteries have a thick middle layer of smooth muscle They have an inner and outer layer of elastic fibres Elastic -

Skeleton-Vasculature Chain Reaction: a Novel Insight Into the Mystery of Homeostasis
Bone Research www.nature.com/boneres REVIEW ARTICLE OPEN Skeleton-vasculature chain reaction: a novel insight into the mystery of homeostasis Ming Chen1,2,YiLi1,2, Xiang Huang1,2,YaGu1,2, Shang Li1,2, Pengbin Yin 1,2, Licheng Zhang1,2 and Peifu Tang 1,2 Angiogenesis and osteogenesis are coupled. However, the cellular and molecular regulation of these processes remains to be further investigated. Both tissues have recently been recognized as endocrine organs, which has stimulated research interest in the screening and functional identification of novel paracrine factors from both tissues. This review aims to elaborate on the novelty and significance of endocrine regulatory loops between bone and the vasculature. In addition, research progress related to the bone vasculature, vessel-related skeletal diseases, pathological conditions, and angiogenesis-targeted therapeutic strategies are also summarized. With respect to future perspectives, new techniques such as single-cell sequencing, which can be used to show the cellular diversity and plasticity of both tissues, are facilitating progress in this field. Moreover, extracellular vesicle-mediated nuclear acid communication deserves further investigation. In conclusion, a deeper understanding of the cellular and molecular regulation of angiogenesis and osteogenesis coupling may offer an opportunity to identify new therapeutic targets. Bone Research (2021) ;9:21 https://doi.org/10.1038/s41413-021-00138-0 1234567890();,: INTRODUCTION cells, pericytes, etc.) secrete angiocrine factors to modulate -

Human Anatomy and Physiology
LECTURE NOTES For Nursing Students Human Anatomy and Physiology Nega Assefa Alemaya University Yosief Tsige Jimma University In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education 2003 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2003 by Nega Assefa and Yosief Tsige All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. Human Anatomy and Physiology Preface There is a shortage in Ethiopia of teaching / learning material in the area of anatomy and physicalogy for nurses. The Carter Center EPHTI appreciating the problem and promoted the development of this lecture note that could help both the teachers and students. -

Structure, Function, and Molecular Control of the Skin Lymphatic System
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector Structure, Function, and Molecular Control of the Skin Lymphatic System Mihaela Skobe and Michael Detmar Cutaneous Biology Research Center, Department of Dermatology, Massachusetts General Hospital and Harvard Medical School, Charlestown, Massachusetts, U.S.A. The mechanisms of angiogenesis have been studied phatic vasculature and the differences between blood extensively over the past years. The focus, however, and lymphatic vessels. Special attention has been has been almost exclusively on blood vessels, whereas given to the methods employed in research of the little effort has been directed toward understanding lymphatic system. Finally, we describe molecular lymphangiogenesis and the role of lymphatic vessels mechanisms involved in the regulation of lymphan- in physiology and pathology. The lymphatic system, giogenesis. Vascular endothelial growth factor and acting in concert with the blood vascular system, is vascular endothelial growth factor-C, expressed by of fundamental importance in maintaining tissue distinct skin cell populations, play an important role homeostasis, and disorders of the lymphatic system in the molecular control of skin angiogenesis and are common, often resulting in chronic, disabling lymphangiogenesis. Key words: lymphatic vessels/lymph- conditions. This overview summarizes the most angiogenesis/skin/VEGF-C. Journal of Investigative important aspects of the structure and function of Dermatology Symposium Proceedings 5:14±19, 2000 the lymphatic system with emphasis on the skin lym- ur understanding of the biology of the lymphatic covered'' (Bartels, 1909). The ancient Greeks observed structures system is well illustrated by the word lymphatic containing colorless ¯uid (Hippocrates spoke of ``white blood'') but itself; the derivation of the latin word lymphaticus their function was not understood and the signi®cance of the signi®es ``distracted and confused'' (Witte et al, ®nding was not recognized. -

Lymphatic Tissue Engineering and Regeneration Laura Alderfer1, Alicia Wei1 and Donny Hanjaya-Putra1,2,3,4,5,6*
Alderfer et al. Journal of Biological Engineering (2018) 12:32 https://doi.org/10.1186/s13036-018-0122-7 REVIEW Open Access Lymphatic Tissue Engineering and Regeneration Laura Alderfer1, Alicia Wei1 and Donny Hanjaya-Putra1,2,3,4,5,6* Abstract The lymphatic system is a major circulatory system within the body, responsible for the transport of interstitial fluid, waste products, immune cells, and proteins. Compared to other physiological systems, the molecular mechanisms and underlying disease pathology largely remain to be understood which has hindered advancements in therapeutic options for lymphatic disorders. Dysfunction of the lymphatic system is associated with a wide range of disease phenotypes and has also been speculated as a route to rescue healthy phenotypes in areas including cardiovascular disease, metabolic syndrome, and neurological conditions. This review will discuss lymphatic system functions and structure, cell sources for regenerating lymphatic vessels, current approaches for engineering lymphatic vessels, and specific therapeutic areas that would benefit from advances in lymphatic tissue engineering and regeneration. Keywords: Lymphangiogenesis, Tissue Engineering, Disease Modeling, Wound Healing, Lymphedema, Stem Cells, Biomaterials, Interstitial Fluid, Regeneration I. Introduction to the Lymphatic System and its role Interstitial fluid (IF) is a plasma filtrate that is generated Function by transcapillary filtration and is governed by Starling The lymphatic system is nearly ubiquitous in the human forces, the net difference between hydrostatic and body, present in all tissues except the epidermis, cartil- osmotic pressures, at the microcirculatory level [9]. In age, eye lens, cornea, retina, and bone marrow [1, 2]. order to maintain fluid homeostasis, lymph formation in The main functions of the lymphatic system include the initial lymphatic vessels must be balanced by the net fluid homeostasis and interstitial fluid drainage, immune flux of plasma being filtered out [4]. -

Coronary Microvascular Dysfunction
Journal of Clinical Medicine Review Coronary Microvascular Dysfunction Federico Vancheri 1,*, Giovanni Longo 2, Sergio Vancheri 3 and Michael Henein 4,5,6 1 Department of Internal Medicine, S.Elia Hospital, 93100 Caltanissetta, Italy 2 Cardiovascular and Interventional Department, S.Elia Hospital, 93100 Caltanissetta, Italy; [email protected] 3 Radiology Department, I.R.C.C.S. Policlinico San Matteo, 27100 Pavia, Italy; [email protected] 4 Institute of Public Health and Clinical Medicine, Umea University, SE-90187 Umea, Sweden; [email protected] 5 Department of Fluid Mechanics, Brunel University, Middlesex, London UB8 3PH, UK 6 Molecular and Nuclear Research Institute, St George’s University, London SW17 0RE, UK * Correspondence: [email protected] Received: 10 August 2020; Accepted: 2 September 2020; Published: 6 September 2020 Abstract: Many patients with chest pain undergoing coronary angiography do not show significant obstructive coronary lesions. A substantial proportion of these patients have abnormalities in the function and structure of coronary microcirculation due to endothelial and smooth muscle cell dysfunction. The coronary microcirculation has a fundamental role in the regulation of coronary blood flow in response to cardiac oxygen requirements. Impairment of this mechanism, defined as coronary microvascular dysfunction (CMD), carries an increased risk of adverse cardiovascular clinical outcomes. Coronary endothelial dysfunction accounts for approximately two-thirds of clinical conditions presenting with symptoms and signs of myocardial ischemia without obstructive coronary disease, termed “ischemia with non-obstructive coronary artery disease” (INOCA) and for a small proportion of “myocardial infarction with non-obstructive coronary artery disease” (MINOCA). More frequently, the clinical presentation of INOCA is microvascular angina due to CMD, while some patients present vasospastic angina due to epicardial spasm, and mixed epicardial and microvascular forms. -

Lymph and Lymphatic Vessels
Cardiovascular System LYMPH AND LYMPHATIC VESSELS Venous system Arterial system Large veins Heart (capacitance vessels) Elastic arteries Large (conducting lymphatic vessels) vessels Lymph node Muscular arteries (distributing Lymphatic vessels) system Small veins (capacitance Arteriovenous vessels) anastomosis Lymphatic Sinusoid capillary Arterioles (resistance vessels) Postcapillary Terminal arteriole venule Metarteriole Thoroughfare Capillaries Precapillary sphincter channel (exchange vessels) Copyright © 2010 Pearson Education, Inc. Figure 19.2 Regional Internal jugular vein lymph nodes: Cervical nodes Entrance of right lymphatic duct into vein Entrance of thoracic duct into vein Axillary nodes Thoracic duct Cisterna chyli Aorta Inguinal nodes Lymphatic collecting vessels Drained by the right lymphatic duct Drained by the thoracic duct (a) General distribution of lymphatic collecting vessels and regional lymph nodes. Figure 20.2a Lymphatic System Outflow of fluid slightly exceeds return Consists of three parts 1. A network of lymphatic vessels carrying lymph 1. Transports fluid back to CV system 2. Lymph nodes 1. Filter the fluid within the vessels 3. Lymphoid organs 1. Participate in disease prevention Lymphatic System Functions 1. Returns interstitial fluid and leaked plasma proteins back to the blood 2. Disease surveillance 3. Lipid transport from intestine via lacteals Venous system Arterial system Heart Lymphatic system: Lymph duct Lymph trunk Lymph node Lymphatic collecting vessels, with valves Tissue fluid Blood Lymphatic capillaries Tissue cell capillary Blood Lymphatic capillaries capillaries (a) Structural relationship between a capillary bed of the blood vascular system and lymphatic capillaries. Filaments anchored to connective tissue Endothelial cell Flaplike minivalve Fibroblast in loose connective tissue (b) Lymphatic capillaries are blind-ended tubes in which adjacent endothelial cells overlap each other, forming flaplike minivalves.