Skeleton-Vasculature Chain Reaction: a Novel Insight Into the Mystery of Homeostasis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arterial and Venous Adaptations to Short-Term Handgrip Exercise Training
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2003 Arterial and venous adaptations to short-term handgrip exercise training Mahmoud Awad Alomari Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Kinesiology Commons Recommended Citation Alomari, Mahmoud Awad, "Arterial and venous adaptations to short-term handgrip exercise training" (2003). LSU Doctoral Dissertations. 188. https://digitalcommons.lsu.edu/gradschool_dissertations/188 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. ARTERIAL AND VENOUS ADAPTATIONS TO SHORT-TERM HANDGRIP EXERCISE TRAINING A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Kinesiology By Mahmoud Alomari B.S., Yarmouk University, Irbid, Jordan, 1990 M.S. Minnesota State University, Mankato, MN, 1995 December, 2003 © Copyright 2003 Mahmoud A. Alomari All right reserved ii DEDICATION I dedicate all of my work to my parents, the love of my life. They feel as though they took every exam with me and were as anxious as I was for each defense. Their confidence in me never wavered and helped me to accomplish the dream of my life. Their motivation made me a better person and they continue to show me what service to others really is. -

Machine-Learning-Based Functional Microcirculation Analysis
The Thirty-Second Innovative Applications of Artificial Intelligence Conference (IAAI-20) Machine-Learning-Based Functional Microcirculation Analysis Ossama Mahmoud1, GH Janssen2,3, Mahmoud R. El-Sakka1 1 Department of Computer Sciences, Western University, London (ON), Canada 2 Department of Medical Biophysics, Western University, London (ON), Canada 3 Centre for Critical Illness Research, Lawson Health Research Institute, London (ON), Canada Abstract The functionality of these microvessels, i.e., the ability to Analysis of microcirculation is an important clinical and re- carry blood flow, has significant implications for organ search task. Functional analysis of the microcirculation al- function during disease progression and overall health. As lows researchers to understand how blood flowing in a tis- such, IVM is used in various medical domains to examine sues’ smallest vessels affects disease progression, organ the microcirculation and to understand disease processes function, and overall health. Current methods of manual analysis of microcirculation are tedious and time- and their effects on the microcirculation (Ellis 2005; consuming, limiting the quick turnover of results. There has Lawendy 2016; Yeh 2017). been limited research on automating functional analysis of The quality of a tissue’s microcirculation can be meas- microcirculation. As such, in this paper, we propose a two- ured through its vascular density. Usually, vascular density step machine-learning-based algorithm to functionally as- for a region of tissue is determined by taking the total sess microcirculation videos. The first step uses a modified vessel segmentation algorithm to extract the location of ves- number of flowing microvessels across a cross-section sel-like structures. While the second step uses a 3D-CNN to divided by the surface area of the region examined (Charl- assess whether the vessel-like structures contained flowing ton 2017). -

Name: Ezenwigbo Johnpaul Oluchukwu College
NAME: EZENWIGBO JOHNPAUL OLUCHUKWU COLLEGE: MEDICINE AND HEALTH SCIENCES DEPARTMENT: MEDICINE AND SURGERY MATRICULATION NUMBER: 18/MHS01/157 COURSE: PHYSIOLOGY LEVEL: 200 LEVEL ASSIGNMENT 1. Discuss the long-term regulation of mean arterial blood pressure? LONG-TERM REGULATION OF MEAN ARTERIAL BLOOD PRESSURE Kidneys play an important role in the long•term regulation of arterial blood pressure. When blood pressure alters slowly in several days/months/years, the nervous mechanism adapts to the altered pressure and loses the sensitivity for the changes. It cannot regulate the pressure any more. In such conditions, the renal mechanism operates efficiently to regulate the blood pressure. Therefore, it is called long•term regulation. Kidneys regulate arterial blood pressure by two ways: A. By regulation of extracellular fluid (ECF) volume B. Through renin•angiotensin mechanism. A. REGULATION OF EXTRACELLULAR FLUID VOLUME: When the blood pressure increases, kidneys excrete large amounts of water and salt, particularly sodium, by means of pressure diuresis and pressure natriuresis. Pressure diuresis is the excretion of large quantity of water in urine because of increased blood pressure. Even a slight increase in blood pressure doubles the water excretion. Pressure natriuresis is the excretion of large quantity of sodium in urine. Because of diuresis and natriuresis, there is a decrease in ECF volume and blood volume, which in turn brings the arterial blood pressure back to normal level. When blood pressure decreases, the reabsorption of water from renal tubules is increased. This in turn, increases ECF volume, blood volume and cardiac output, resulting in restoration of blood pressure. B. THROUGH RENIN-ANGIOTENSIN MECHANISM: When blood pressure and ECF volume decrease, renin secretion from kidneys is increased. -

7Th & 8 March-2016- Papers of All Specialties (1705 MCQS)
1 7th & 8th March-2016- Papers of all Specialties (1705 MCQS) [ Index- Check List ] Compiled by : Amlodipine Besylate (1) Medicine & Allied 7th March 2016 (Evening Session) by Alizay Khan (181 MCQS) Page#1 (2) Medice & Allied 8th March 2016 (Morning Session) by Dr Kunza Aslam (200 MCQS) P#15 (3) Medicine 8th March(Evening) by Dr.Tariq Khan/Mudassir Bangash (200MCQS) P#29 (4).Surgery & Allied 7th March (Evening Session) by Dr. Hasnain Afzal (197 MCQS) P#40 (5). Surgery & Allied 7th March (Evening Session) - by Dr.Xaheer Khan (185 MCQS) P#57 (6). Surgery 7th March 2016 (Morning Session) by By Dr.Haris Riaz Sheikh (156+) P#73 (7). Gyane/Obs 7th March-2016 (Morning Session) by Dr.Noor Fatima (184) P#89 (8)..Gynae / Obs; 8th March 2016 (Morning Session) Dr.Nourin Hameed (105) P#94 (9). Radiology 7th March-2016(Morning) by Dr.Asfandyar Khan Bhittani & Loa Loa(122) P#103 (10). Community Medicine 7th March 2016 (Morning) by Dr.Qaisar Javed (90+85) P#112 =-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-=-= (1)Medicine & Allied 7th March 2016(evening) by Alizay Khan (181) 1. anterior cruciate ligament is damaged.direction of tibial dislocation on femur is a. anteriolateral b. anteromeddiaal c. anterior (answer) d. posterromedial e. posterolateral 2. narrowest point in pediatric airway a. cricoid (answer) b. thyroid c. trachea d. false vocal cord e. true vocal cords 3. regarding vertebral column a. intervertebral disc is thickest in thoracic and lumber regions b. cervical vertebrae are 7(answer) c. total 31 vertebrae d. curvature to side is caalled lordosis e. prolapse can occur without fracutre 4. -

Skeletal System
Skeletal System Overview • The skeletal system composed of bones, cartilages, joints, and ligaments, accounts for about 20% of the body mass (i.e., about 30 pounds in a 160-pound person). o Bones make up most of the skeleton o Cartilages occur only in isolated areas, such as the nose, parts of ribs, and the joints o Ligaments connect bones and reinforce joints, allowing required movements while restricting motions in other directions. o Joints are the junctions between bones which provide for the mobility of the skeleton Skeletal Cartilages • Human skeleton initially made up of cartilages and fibrous membranes; most are soon replaced with bone • In adults, the few areas where cartilage remains are mainly where flexible skeletal tissue is needed. • Cartilage tissue consists mainly of water—approximately 80%; high water content allows cartilage to be resilient (i.e., spring back to its original shape after being compressed). • Cartilage contains no nerves or blood vessels. • Perichondrium (“around the cartilage”) is dense irregular connective tissue; surrounds the cartilage and acts like a girdle to resist outward expansion when cartilage is compressed. o Perichondrium contains the blood vessels from which nutrients diffuse through the matrix to reach the cartilage cells. This mode of nutrient delivery limits cartilage thickness. • Three types of Cartilage Tissue in body o All three have cells called chondrocytes encased in small cavities (called lacunae) within an extracellular matrix containing a jellylike ground substance and fibers. o Skeletal cartilages contain representatives from all three types. Hyaline cartilages • Looks like frosted glass • Most abundant skeletal cartilages • Their chondrocytes appear spherical • Only fiber type in their matrix is fine collagen (undetectable microscopically) • Skeletal hyaline cartilages include: o Articular Cartilages —cover ends of most bones at movable joints o Costal cartilages —connect ribs to sternum o Respiratory cartilages —form skeleton of the larynx (voicebox) and reinforce other respiratory passages. -

Coronary Microvascular Dysfunction
Journal of Clinical Medicine Review Coronary Microvascular Dysfunction Federico Vancheri 1,*, Giovanni Longo 2, Sergio Vancheri 3 and Michael Henein 4,5,6 1 Department of Internal Medicine, S.Elia Hospital, 93100 Caltanissetta, Italy 2 Cardiovascular and Interventional Department, S.Elia Hospital, 93100 Caltanissetta, Italy; [email protected] 3 Radiology Department, I.R.C.C.S. Policlinico San Matteo, 27100 Pavia, Italy; [email protected] 4 Institute of Public Health and Clinical Medicine, Umea University, SE-90187 Umea, Sweden; [email protected] 5 Department of Fluid Mechanics, Brunel University, Middlesex, London UB8 3PH, UK 6 Molecular and Nuclear Research Institute, St George’s University, London SW17 0RE, UK * Correspondence: [email protected] Received: 10 August 2020; Accepted: 2 September 2020; Published: 6 September 2020 Abstract: Many patients with chest pain undergoing coronary angiography do not show significant obstructive coronary lesions. A substantial proportion of these patients have abnormalities in the function and structure of coronary microcirculation due to endothelial and smooth muscle cell dysfunction. The coronary microcirculation has a fundamental role in the regulation of coronary blood flow in response to cardiac oxygen requirements. Impairment of this mechanism, defined as coronary microvascular dysfunction (CMD), carries an increased risk of adverse cardiovascular clinical outcomes. Coronary endothelial dysfunction accounts for approximately two-thirds of clinical conditions presenting with symptoms and signs of myocardial ischemia without obstructive coronary disease, termed “ischemia with non-obstructive coronary artery disease” (INOCA) and for a small proportion of “myocardial infarction with non-obstructive coronary artery disease” (MINOCA). More frequently, the clinical presentation of INOCA is microvascular angina due to CMD, while some patients present vasospastic angina due to epicardial spasm, and mixed epicardial and microvascular forms. -
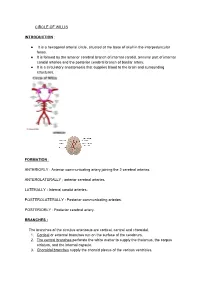
CIRCLE of WILLIS INTRODUCTION: It Is a Hexagonal
CIRCLE OF WILLIS INTRODUCTION : ● It is a hexagonal arterial circle, situated at the base of skull in the interpeduncular fossa. ● It is formed by the anterior cerebral branch of internal carotid, terminal part of internal carotid arteries and the posterior cerebral branch of basilar artery. ● It is a circulatory anastomosis that supplies blood to the brain and surrounding structures. FORMATION : ANTERIORLY : Anterior communicating artery joining the 2 cerebral arteries. ANTEROLATERALLY : anterior cerebral arteries. LATERALLY : Internal carotid arteries. POSTEROLATERALLY : Posterior communicating arteries. POSTERIORLY : Posterior cerebral artery. BRANCHES : The branches of the circulus arteriosus are cortical, central and choroidal. 1. Cortical or external branches run on the surface of the cerebrum. 2. The central branches perforate the white matter to supply the thalamus, the corpus striatum, and the internal capsule. 3. Choroidal branches supply the choroid plexus of the various ventricles. CORTICAL BRANCHES : These branches arise from all three cerebral arteries: ● Anterior cerebral ● Middle cerebral ● Posterior cerebral. 1. MIDDLE CEREBRAL ARTERY : It is the direct branch of internal carotid artery. CORTICAL BRANCHES : ● orbital ● Frontal ● Parietal ● Temporal. 2. ANTERIOR CEREBRAL ARTERY : It Is the smallest terminal branch of internal carotid artery. CORTICAL BRANCHES : ● Orbital ● Frontal ● Parietal. 3. POSTERIOR CEREBRAL : It is the terminal branch of basilar artery. CORTICAL BRANCHES : ● Temporal ● Occipital ● Parieto occipital. CEREBRAL CORTEX : Cerebral cortex is supplied by all the 3 arteries. ● SUPEROLATERAL SURFACE : This surface is mainly supplied by middle cerebral artery. ● MEDICAL AND TENTORIAL SURFACE : This surface is supplied by anterior cerebral artery. ● INFERIOR : Medial one third of orbital surface is supplied by anterior cerebral. Lateral two third, including the Temporal pole area and anterior surface of the Temporal pole is vascularised by middle cerebral artery. -

Review Article Structure and Functions of Blood Vessels and Vascular Niches in Bone
Hindawi Stem Cells International Volume 2017, Article ID 5046953, 10 pages https://doi.org/10.1155/2017/5046953 Review Article Structure and Functions of Blood Vessels and Vascular Niches in Bone 1,2 Saravana K. Ramasamy 1Institute of Clinical Sciences, Imperial College London, London W12 0NN, UK 2MRC London Institute of Medical Sciences, Imperial College London, London W12 0NN, UK Correspondence should be addressed to Saravana K. Ramasamy; [email protected] Received 5 May 2017; Revised 26 July 2017; Accepted 23 August 2017; Published 17 September 2017 Academic Editor: Hong Qian Copyright © 2017 Saravana K. Ramasamy. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Bone provides nurturing microenvironments for an array of cell types that coordinate important physiological functions of the skeleton, such as energy metabolism, mineral homeostasis, osteogenesis, and haematopoiesis. Endothelial cells form an intricate network of blood vessels that organises and sustains various microenvironments in bone. The recent identification of heterogeneity in the bone vasculature supports the existence of multiple vascular niches within the bone marrow compartment. A unique combination of cells and factors defining a particular microenvironment, supply regulatory signals to mediate a specific function. This review discusses recent developments in our understanding of vascular niches in bone that play a critical role in regulating the behaviour of multipotent haematopoietic and mesenchymal stem cells during development and homeostasis. 1. Introduction Blood vessels in bone are reported to provide nurturing microenvironments to haematopoietic stem cells (HSCs) Recent advancements in vascular biology have increased our [21, 22] and mesenchymal stem cells (MSCs) [23, 24]. -

Physiology As
NAME: OKE ANUOLUWAPO ENIOLA MATRIC NUMBER: 18/MHS01/262 LEVEL: 200 LEVEL DEPARTMENT: MEDICINE AND SURGERY COURSE: PHYSIOLOGY ASSIGNMENT: 1) DISSCUSS THE LONG TERM REGULATION OF MEAN ARTERIAL BLOOD PRESSURE 2) WRITE SHORT NOTES ON A) PULMONARY CIRCULATION B) CIRCLE OF WILLIS C) SPLANCHNIC CIRCULATION D) CORONARY CIRCULATIOM E) CUTANEOUS CIRCULATION 3) DISCUSS THE CARDIOVASCULAR ADJUSTMENT THAT OCCURS DURING EXERCISE 1) DISCUSS THE LONG-TERM REGULATION OF MEAN ARTERIAL BLOOD RESSURE Arterial blood pressure varies even under physiological conditions. However, it’s immediately brought back to normal level because of the presence of well- organized regulatory mechanisms in the body. One of those mechanisms is the long term regulatory mechanism (renal mechanism). LONG TERM REGULATORY MECHANISM (RENAL MECHANISM) Consistent and long term control of blood-pressure is determined by the renin- angiotensin system. Along with vessel morphology, blood viscosity is one of the key main factors influencing resistance and hence blood pressure. A key modulator of blood pressure is the Renin-Angiotensin System (RAS) or the renin Angiotensin-Aldosterone System (RAAS), a hormone system that regulates blood pressure and water balance. When the volume of blood is low, juxtaglomerular cells in the kidney secrete renin directly into circulation. Plasma renin then carries out the conversion of angiotensinogen released by the liver to angiotensin I. Angiotensin I is subsequently converted to angiotensin II by the enzyme found in the lungs. Angiotensin II is a potent vasoactive peptide that causes blood vessels to constrict, resulting in increased blood pressure. Angiotensin II also stimulates the secretion of the hormone aldosterone from the adrenal cortex. -

The Microcirculation of the Mammalian Lymph Node
155 Lymphology 9 (1976) 155- 157 © Georg Thieme Verlag Stuttgart The Microcirculation of the Mammalian Lymph Node 8 .8. Hobbs, J.W. Davidson Radiological Research Laboratories University of Toronto, Toronto, Ontario, Canada Summary Flow alterations to give complete filling of the lymphatic sinusoidal system and saccular lymph spaces around the germinal centers were demonstrated during a primary immune reaction. By contrast, in delayed hyper· sensitivity, saccules were not seen although there was marked enlargement of individual fo llicular units. The vascular and lymphatic microcirculations of the popliteal lymph node of normal adult ew Zealand white rabbits were studied following injections of micro fit * into afferent arteries and lymphatics. Vessels and lymphatic spaces within the lymph nodes of normal antigenically ex· perienced animals were compared with those regional to an injection of the antigen Keyhole Limpet Hemocyanin** 2 mgs. In a third group of animals previously sensitized to killed tuber cle bacilli, a challenging dose of purified protein derivative of old tuberculin was given, and both microcirculations studied after an interval of 48 hours. In normal animals, afferent lymph vessels lead to a dome shaped network of sinusoids around individual follicles. 1l1cse continue di rectly into a dense medullary sinusoidal network leading in tum to small efferent canaliculi and large calibre efferent trunks. Flow of the casting medium from afferent to efferent lymphatics frequently occurred only through a segment of the lymph node with non filling of many adjacent areas. Within individual cleared sub marginal follicles, a few small circumscribed saccular collections were demonstrated (Fig. I). ,-- I • J .· Fig. I Radiograph x 20 of Microfil withi n a normal popliteal node foll owing intralymphatic injection. -

Role of the Renal Microcirculation in Antihypertensive Therapy
REVIEW ICME CREDIT \ Role of the renal microcirculation in antihypertensive therapy SHARON R. 1NMAN, PHD; NICHOLAS T. STOWE, PHD; DONALD G. VIDT, MD BACKGROUND The renal circulation plays a central role in regulating blood pressure and glomerular filtration. YPERTENSION IS OBJECTIVE To examine the effects of the various classes Hcharacterized by an of antihypertensive agents on the renal microcirculation. increase in peripheral vascular resistance, SUMMARY Peripheral vascular resistance is generally increased in generally in proportion to the hypertension, and the microcirculation makes the major contribution elevation in blood pressure. In to resistance. In the kidney, the preglomerular and postglomerular ves- the early stages of hypertension, sels constrict to protect the glomerular capillary from increased hydro- the increase in resistance is lim- static pressure, further increasing peripheral resistance. Because the ited to the kidney; in the later renal microcirculation adjusts to maintain glomerular filtration and stages the increase is shared by blood flow, antihypertensive agents that can normalize the pressure most organ systems.1 This re- and blood flow in these vessels may help prevent the long-term conse- sponse is thought to occur in the quences of hypertension. Angiotensin-Converting enzyme inhibitors resistance vessels 23; the largest, directly affect preglomerular and postglomerular resistance, but they rather than the smallest arteri- further decrease postglomerular resistance. Calcium antagonists selec- oles, make the greatest contribu- tively decrease preglomerular resistance. The diuretics, vasodilators, al- tion to resistance,4 both in hyper- pha blockers, and beta blockers may also cause changes in tension and in normal blood preglomerular and postglomerular resistance; however, compensatory pressure. reflex responses may mitigate their direct effects. -

The Transcortical Vessel Is Replacement of Cortical Capillary Or a Separate Identity in Diaphyseal Vascularity
Letter to Editor https://doi.org/10.5115/acb.19.171 pISSN 2093-3665 eISSN 2093-3673 The transcortical vessel is replacement of cortical capillary or a separate identity in diaphyseal vascularity Adil Asghar1, Ravi Kant Narayan1, Ashutosh Kumar1, Shagufta Naaz2 Deparments of 1Anatomy and 2Anaesthesiology, All India Institute of Medical Sciences, Patna, India Received August 5, 2019; Revised October 5, 2019; Accepted October 7, 2019 A recent article published in Nature Metabolism “A net- cow and imagined that bone had small pipes going long ways. work of trans-cortical capillaries as a mainstay for blood Since the last four centuries, we were baffling about diaphy- circulation in long bones” by Grüneboom et al. (2019) [1] seal vascularity and niche of hemopoietic cells. The contro- is a remarkable description of the bone-vascular network. versies exist from the era of Clapton Havers (1691) [3] who The discovery of transcortical vessels (TCVs) in long bones discovered nutrient artery. Albinus Bernharbiner et al. (1754) has witnessed the enigma of bone vascularity. In the mouse [4] proposed the centrifugal vascularity of cortical bone by model, they showed hundreds of TCVs originating from tiny vessels running in a canal along the long axis of shaft bone marrow which travels the whole cortical thickness. They named as Haversian canals, while the oblique or transverse claimed these TCVs to be the same as seen in the human tibia canal was named Volkmann’s canal later on. The perfusion and femoral epiphysis. TCVs express arterial or venous mark- techniques like Barium sulfate and Indian ink etc. delineated ers, hence are the mainstay of bone vascularity because 80% the three major parts of circulation in bone—medullary ves- of arterial and 59% of venous blood passes through them [1].