Effects of Spinosad and Lambda-Cyhalothrin on Their
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Vol. 16, No. 2 Summer 1983 the GREAT LAKES ENTOMOLOGIST
MARK F. O'BRIEN Vol. 16, No. 2 Summer 1983 THE GREAT LAKES ENTOMOLOGIST PUBLISHED BY THE MICHIGAN EN1"OMOLOGICAL SOCIErry THE GREAT LAKES ENTOMOLOGIST Published by the Michigan Entomological Society Volume 16 No.2 ISSN 0090-0222 TABLE OF CONTENTS Seasonal Flight Patterns of Hemiptera in a North Carolina Black Walnut Plantation. 7. Miridae. J. E. McPherson, B. C. Weber, and T. J. Henry ............................ 35 Effects of Various Split Developmental Photophases and Constant Light During Each 24 Hour Period on Adult Morphology in Thyanta calceata (Hemiptera: Pentatomidae) J. E. McPherson, T. E. Vogt, and S. M. Paskewitz .......................... 43 Buprestidae, Cerambycidae, and Scolytidae Associated with Successive Stages of Agrilus bilineatus (Coleoptera: Buprestidae) Infestation of Oaks in Wisconsin R. A. Haack, D. M. Benjamin, and K. D. Haack ............................ 47 A Pyralid Moth (Lepidoptera) as Pollinator of Blunt-leaf Orchid Edward G. Voss and Richard E. Riefner, Jr. ............................... 57 Checklist of American Uloboridae (Arachnida: Araneae) Brent D. Ope II ........................................................... 61 COVER ILLUSTRATION Blister beetles (Meloidae) feeding on Siberian pea-tree (Caragana arborescens). Photo graph by Louis F. Wilson, North Central Forest Experiment Station, USDA Forest Ser....ice. East Lansing, Michigan. THE MICHIGAN ENTOMOLOGICAL SOCIETY 1982-83 OFFICERS President Ronald J. Priest President-Elect Gary A. Dunn Executive Secretary M. C. Nielsen Journal Editor D. C. L. Gosling Newsletter Editor Louis F. Wilson The Michigan Entomological Society traces its origins to the old Detroit Entomological Society and was organized on 4 November 1954 to " ... promote the science ofentomology in all its branches and by all feasible means, and to advance cooperation and good fellowship among persons interested in entomology." The Society attempts to facilitate the exchange of ideas and information in both amateur and professional circles, and encourages the study of insects by youth. -

Research Article Ecological Observations of Native Geocoris Pallens and G
Hindawi Publishing Corporation Psyche Volume 2013, Article ID 465108, 11 pages http://dx.doi.org/10.1155/2013/465108 Research Article Ecological Observations of Native Geocoris pallens and G. punctipes Populations in the Great Basin Desert of Southwestern Utah Meredith C. Schuman, Danny Kessler, and Ian T. Baldwin Department of Molecular Ecology, Max Planck Institute for Chemical Ecology, Hans-Knoll-Straße¨ 8, 07745 Jena, Germany Correspondence should be addressed to Ian T. Baldwin; [email protected] Received 5 November 2012; Accepted 16 April 2013 Academic Editor: David G. James Copyright © 2013 Meredith C. Schuman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Big-eyed bugs (Geocoris spp. Fallen,´ Hemiptera: Lygaeidae) are ubiquitous, omnivorous insect predators whose plant feeding behavior raises the question of whether they benefit or harm plants. However, several studies have investigated both the potential of Geocoris spp. to serve as biological control agents in agriculture and their importance as agents of plant indirect defense in nature. These studies have demonstrated that Geocoris spp. effectively reduce herbivore populations and increase plant yield. Previous work has also indicated that Geocoris spp. respond to visual and olfactory cues when foraging and choosing their prey and that associative learning of prey and plant cues informs their foraging strategies. For these reasons, Geocoris spp. have become models for the study of tritrophic plant-herbivore-predator interactions. Here, we present detailed images and ecological observations of G. pallens Stal˚ and G. -

A Comparative Study of Two Seed Bugs, Geocoris
A COMPARATIVE STUDY OF TWO SEED BUGS, GEOCORIS BULLATUS (SAY) AND G. DISCOPTERUS STAL (HEMIPTERA: LYGAEIDAE) IN THE YUKON. By JENNIFER J. ROBINSON B.Sc. Trent University, 1980 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in THE FACULTY OF GRADUATE STUDIES DEPARTMENT OF ZOOLOGY We accept this thesis as conforming te trie required standard June, 1985 (c) Jennifer J. Robinson, 1985 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department of The University of British Columbia 1956 Main Mall Vancouver, Canada V6T 1Y3 )E-6 C3/81) Abstract Geocoris bullatus (Say 1831), (Henriptera: Lygaeidae) has been collected and studied across North America but the present work is the o first detailed study of western North American CL discopterus Stal 1874. In fact, it has been claimed that 6^. discopterus is solely a species of the east. As the two species are taxonomically difficult to separate, when they were apparently discovered together at several localities in the southwestern Yukon, a detailed investigation of their systematics and distribution seemed necessary. Species status of Yukon Q. bullatus and iG. -

Insecticides - Development of Safer and More Effective Technologies
INSECTICIDES - DEVELOPMENT OF SAFER AND MORE EFFECTIVE TECHNOLOGIES Edited by Stanislav Trdan Insecticides - Development of Safer and More Effective Technologies http://dx.doi.org/10.5772/3356 Edited by Stanislav Trdan Contributors Mahdi Banaee, Philip Koehler, Alexa Alexander, Francisco Sánchez-Bayo, Juliana Cristina Dos Santos, Ronald Zanetti Bonetti Filho, Denilson Ferrreira De Oliveira, Giovanna Gajo, Dejane Santos Alves, Stuart Reitz, Yulin Gao, Zhongren Lei, Christopher Fettig, Donald Grosman, A. Steven Munson, Nabil El-Wakeil, Nawal Gaafar, Ahmed Ahmed Sallam, Christa Volkmar, Elias Papadopoulos, Mauro Prato, Giuliana Giribaldi, Manuela Polimeni, Žiga Laznik, Stanislav Trdan, Shehata E. M. Shalaby, Gehan Abdou, Andreia Almeida, Francisco Amaral Villela, João Carlos Nunes, Geri Eduardo Meneghello, Adilson Jauer, Moacir Rossi Forim, Bruno Perlatti, Patrícia Luísa Bergo, Maria Fátima Da Silva, João Fernandes, Christian Nansen, Solange Maria De França, Mariana Breda, César Badji, José Vargas Oliveira, Gleberson Guillen Piccinin, Alan Augusto Donel, Alessandro Braccini, Gabriel Loli Bazo, Keila Regina Hossa Regina Hossa, Fernanda Brunetta Godinho Brunetta Godinho, Lilian Gomes De Moraes Dan, Maria Lourdes Aldana Madrid, Maria Isabel Silveira, Fabiola-Gabriela Zuno-Floriano, Guillermo Rodríguez-Olibarría, Patrick Kareru, Zachaeus Kipkorir Rotich, Esther Wamaitha Maina, Taema Imo Published by InTech Janeza Trdine 9, 51000 Rijeka, Croatia Copyright © 2013 InTech All chapters are Open Access distributed under the Creative Commons Attribution 3.0 license, which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. After this work has been published by InTech, authors have the right to republish it, in whole or part, in any publication of which they are the author, and to make other personal use of the work. -

2005 Project Abstract for the Period Ending June 30, 2008 PROJECT
2005 Project Abstract For the Period Ending June 30, 2008 PROJECT TITLE: Biological Control of European Buckthorn and Garlic Mustard PROJECT MANAGER: Luke Skinner AFFILIATION: Minnesota Department of Natural Resources MAILING ADDRESS: 500 Lafayette Road Box 25 CITY/STATE/ZIP: St. Paul MN 55155 PHONE: 651-259-5140 FAX: 651-296-1811 E-MAIL: [email protected] WEBSITE: (If applicable) FUNDING SOURCE: Minnesota Environment and Natural Resources Trust Fund LEGAL CITATION: [ML 2005, First Special Session, [Chap. 1], Art. 2, Sec.[11], Subd. 5 (h).] APPROPRIATION AMOUNT: $200,000 Overall Project Outcome and Results This project builds upon and continues work begun from a 2003 Trust Fund appropriation and has since received an additional 2007 Trust Fund appropriation to further continue and accelerate the work. Buckthorn and garlic mustard are invasive species of highest priority for development of long- term management solutions, such as biological control (bio-control). This research aimed to help determine 1) if there are suitable insects that can be used to reduce impacts caused by buckthorn and 2) to implement introduction of insects to control garlic mustard and assess their establishment and success. Buckthorn. Insects were collected and reared for carrying out host specificity testing. A total of 1,733 specimens (356 species) were collected from buckthorn infestations in this insect fauna survey. In total, 39 specialized arthopods were recorded from R. cathartica (common buckthorn) and F. alnus (glossy buckthorn) in Europe. The reassessment of the potential for biological control of R. cathartica and F. alnus was conducted based on work done in Europe from 2002-2007 on potential biological control agents. -

Biological Control of Insect Pests in the Tropics - M
TROPICAL BIOLOGY AND CONSERVATION MANAGEMENT – Vol. III - Biological Control of Insect Pests In The Tropics - M. V. Sampaio, V. H. P. Bueno, L. C. P. Silveira and A. M. Auad BIOLOGICAL CONTROL OF INSECT PESTS IN THE TROPICS M. V. Sampaio Instituto de Ciências Agrária, Universidade Federal de Uberlândia, Brazil V. H. P. Bueno and L. C. P. Silveira Departamento de Entomologia, Universidade Federal de Lavras, Brazil A. M. Auad Embrapa Gado de Leite, Empresa Brasileira de Pesquisa Agropecuária, Brazil Keywords: Augmentative biological control, bacteria, classical biological control, conservation of natural enemies, fungi, insect, mite, natural enemy, nematode, predator, parasitoid, pathogen, virus. Contents 1. Introduction 2. Natural enemies of insects and mites 2.1. Entomophagous 2.1.1. Predators 2.1.2. Parasitoids 2.2. Entomopathogens 2.2.1. Fungi 2.2.2. Bacteria 2.2.3. Viruses 2.2.4. Nematodes 3. Categories of biological control 3.1. Natural Biological Control 3.2. Applied Biological Control 3.2.1. Classical Biological Control 3.2.2. Augmentative Biological Control 3.2.3. Conservation of Natural Enemies 4. Conclusions Glossary UNESCO – EOLSS Bibliography Biographical Sketches Summary SAMPLE CHAPTERS Biological control is a pest control method with low environmental impact and small contamination risk for humans, domestic animals and the environment. Several success cases of biological control can be found in the tropics around the world. The classical biological control has been applied with greater emphasis in Australia and Latin America, with many success cases of exotic natural enemies’ introduction for the control of exotic pests. Augmentative biocontrol is used in extensive areas in Latin America, especially in the cultures of sugar cane, coffee, and soybeans. -

Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects
EN58CH06-Casida ARI 5 December 2012 8:11 Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects John E. Casida1,∗ and Kathleen A. Durkin2 1Environmental Chemistry and Toxicology Laboratory, Department of Environmental Science, Policy, and Management, 2Molecular Graphics and Computational Facility, College of Chemistry, University of California, Berkeley, California 94720; email: [email protected], [email protected] Annu. Rev. Entomol. 2013. 58:99–117 Keywords The Annual Review of Entomology is online at acetylcholinesterase, calcium channels, GABAA receptor, nicotinic ento.annualreviews.org receptor, secondary targets, sodium channel This article’s doi: 10.1146/annurev-ento-120811-153645 Abstract Copyright c 2013 by Annual Reviews. Neuroactive insecticides are the principal means of protecting crops, people, All rights reserved livestock, and pets from pest insect attack and disease transmission. Cur- ∗ Corresponding author rently, the four major nerve targets are acetylcholinesterase for organophos- phates and methylcarbamates, the nicotinic acetylcholine receptor for neonicotinoids, the γ-aminobutyric acid receptor/chloride channel for by Public Health Information Access Project on 04/29/14. For personal use only. Annu. Rev. Entomol. 2013.58:99-117. Downloaded from www.annualreviews.org polychlorocyclohexanes and fiproles, and the voltage-gated sodium channel for pyrethroids and dichlorodiphenyltrichloroethane. Species selectivity and acquired resistance are attributable in part to structural differences in binding subsites, receptor subunit interfaces, or transmembrane regions. Additional targets are sites in the sodium channel (indoxacarb and metaflumizone), the glutamate-gated chloride channel (avermectins), the octopamine receptor (amitraz metabolite), and the calcium-activated calcium channel (diamides). Secondary toxic effects in mammals from off-target serine hydrolase inhibi- tion include organophosphate-induced delayed neuropathy and disruption of the cannabinoid system. -

BOARD of PESTICIDES CONTROL January 15, 2020 Augusta Civic Center, 76 Community Drive, Kennebec/Penobscot Room, Augusta, Maine
STATE OF MAINE DEPARTMENT OF AGRICULTURE, CONSERVATION AND FORESTRY BOARD OF PESTICIDES CONTROL 28 STATE HOUSE STATION UGUSTA AINE JANET T. MILLS A , M 04333 AMANDA E. BEAL GOVERNOR COMMISSIONER BOARD OF PESTICIDES CONTROL January 15, 2020 Augusta Civic Center, 76 Community Drive, Kennebec/Penobscot Room, Augusta, Maine 1:00 - 1:30 PM Board Meeting 1:30 - 2:30 PM Public Forum On Notification 2:30 – 4:00 PM Board Meeting Continued AGENDA 1. Introductions of Board and Staff 2. Minutes of the November 8, 2019 Board Meeting Presentation By: Megan Patterson, Director Action Needed: Amend and/or Approve 3. Request for Financial Support from the Maine Mobile Health Program and the Eastern Maine Development Corporation Since 1995 the Board has supported a Migrant and Seasonal Farmworker Safety Education program. The Maine Mobile Health Program (MMHP) and Eastern Maine Development Corporation (EMDC provided training to 315 migrant agricultural workers during the 2019 season). Funding to support this effort in 2020 is being requested in the amount of $5,360, which is the same amount the Board provided in 2019. The funding has been accounted for in the Board’s FY20 budget. Presentation By: Chris Huh, Program Manager, Farmworkers Jobs Program, Eastern Maine Development Corporation Elizabeth Charles McGough, Director of Outreach, Maine Mobile Health Program MEGAN PATTERSON, DIRECTOR PHONE: (207) 287-2731 90 BLOSSOM LANE, DEERING BUILDING WWW.THINKFIRSTSPRAYLAST.ORG Action Needed: Discussion and Determination if the Board Wishes to Fund this Request 4. Request for Financial Support from the Maine State Apiarist for CLEAR Training Maine State Apiarist, Jennifer Lund, has requested funding to attend the National Certified Investigator & Inspector Basic Training held in Raleigh, North Carolina in March 2020. -

Medication List
Medication List Walgreens Plus™ members receive discounts on thousands of generic and brand-name medications included on this Medication List, which is divided into two sections, “Value Priced” Medications and “Discounted” Medications*. The price for a medication identified as “Value-Priced” is listed below: Get savings up to 85% off Cash Prices • 30-day-supply drugs cost $5 (tier 1), $10 (tier 2) or $15 (tier 3) on Atorvastatin (generic Lipitor) and • 90-day-supply drugs cost $10 (tier 1), $20 (tier 2) or $30 (tier 3) Rosuvastatin (generic Crestor) †† The Discounted Medications section lists the discounts offered to Walgreens Plus members on other generic and brand-name medications not included in the Value-Priced Medication section. The price for a medication is based on its tier and whether it is a 30-day or 90-day supply†. There may be an additional cost for quanities greater than those listed. This discount prescription pricing applies only to Walgreen Plus members on prescriptions purchased in select Walgreens stores that are not billed to insurance and/or used in combination with other health or pharmacy benefit programs. For further details, see your pharmacist or Walgreens.com/Plus. VALUE GENERICS NAPROXEN 250MG TAB 2 60 180 Antifungal NAPROXEN 500MG TAB 2 60 180 Quantity NAPROXEN 375MG TAB 2 60 180 Drug Name Tier 30 90 NAPROXEN DR 500MG TAB 3 60 180 FLUCONAZOLE 150MG TAB 2 1 3 TERBINAFINE 250MG TAB 2 30 90 Asthma Quantity Antiviral Drug Name Tier 30 90 Quantity ALBUTEROL 0.083% INH SOLN 25X3ML 2 75 225 Drug Name Tier 30 90 AMINOPHYLLINE -

Predation of the Chinch Bug, Blissus Occiduus Barber (Hemiptera: Blissidae) by Geocoris Uliginosus (Say) (Hemiptera: Lygaeidae)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications: Department of Entomology Entomology, Department of 2008 Predation of the Chinch Bug, Blissus occiduus Barber (Hemiptera: Blissidae) by Geocoris uliginosus (Say) (Hemiptera: Lygaeidae) J. D. Carstens University of Nebraska-Lincoln Frederick P. Baxendale University of Nebraska-Lincoln, [email protected] Tiffany Heng-Moss University of Nebraska-Lincoln, [email protected] Robert J. Wright University of Nebraska, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/entomologyfacpub Part of the Entomology Commons Carstens, J. D.; Baxendale, Frederick P.; Heng-Moss, Tiffany; and Wright, Robert J., "Predation of the Chinch Bug, Blissus occiduus Barber (Hemiptera: Blissidae) by Geocoris uliginosus (Say) (Hemiptera: Lygaeidae)" (2008). Faculty Publications: Department of Entomology. 157. https://digitalcommons.unl.edu/entomologyfacpub/157 This Article is brought to you for free and open access by the Entomology, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications: Department of Entomology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. JOURNAL OF THE KANSAS ENTOMOLOGICAL SOCIETY 81(4), 2008, pp. 328–338 Predation of the Chinch Bug, Blissus occiduus Barber (Hemiptera: Blissidae) by Geocoris uliginosus (Say) (Hemiptera: Lygaeidae) J. D. CARSTENS,F.P.BAXENDALE,T.M.HENG-MOSS, AND R. J. WRIGHT Department of Entomology, University of Nebraska-Lincoln, Lincoln, NE 68583 ABSTRACT: Big-eyed bugs have been well documented as predators on a diverse group of arthropod prey in turfgrasses; however, little is known about the big-eyed bug species associated with buffalograss, or their feeding habits relative to the western chinch bug, Blissus occiduus Barber. -

Impact of Insecticides Used to Control Spodoptera Frugiperda (J.E
RESEARCH Impact of insecticides used to control Spodoptera frugiperda (J.E. Smith) in corn on survival, sex ratio, and reproduction of Trichogramma pretiosum Riley offspring Jander R. Souza1, Geraldo A. Carvalho1, Alexandre P. Moura2*, Marcelo H.G. Couto1, and Jader B. Maia1 Corn (Zea mays L.) is cultivated in large areas and considered one of the world’s major cereal crops. There are several arthropod pests that can reduce its production such as the fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lep.: Noctuidae), which is considered to be the main pest for corn. Fall armyworm is primarily controlled by insecticides. The use of biological control agents to manage this pest is growing with an emphasis on the egg parasitoid Trichogramma pretiosum Riley (Hym.: Trichogrammatidae). Thus, the aim of this research was to evaluate the impact of the following insecticides (g ai L-1) beta-cypermethrin (0.03), chlorfenapyr (0.60), chlorpyrifos (0.96), spinosad (0.16), etofenprox (0.10), triflumuron (0.08), alfa-cypermethrin/teflubenzuron (0.0425/0.0425), and lambda-cyhalothrin/thiamethoxam (0.11/0.083) on survival, sex ratio, reproduction, and T. pretiosum offspring. Distilled water was used as a control. Commercial insecticide formulations were diluted in distilled water. Bioassays used Anagasta kuehniella eggs treated with insecticides which were afterwards exposed to parasitism. Bioassays were conducted under controlled conditions at 25 ± 2 ºC, 70 ± 10% RH, and 12:12 h photoperiod. Alfa-cypermethrin/teflubenzuron, beta-cypermethrin, chlorpyrifos, chlorfenapyr, spinosad, etofenprox, and lambda-cyhalothrin/thiamethoxam reduced parasitism capacity of maternal generation females as well as the percentage of insect emergence from the F1 generation. -
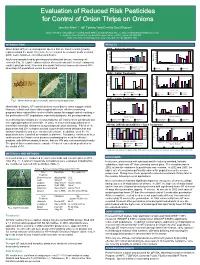
Evaluation of Reduced Risk Pesticides for Control of Onion Thrips on Onions
Evaluation of Reduced Risk Pesticides for Control of Onion Thrips on Onions Jennifer Allen1,3, Jeff Tolman2 and Cynthia Scott-Dupree3 1 – Ontario Ministry of Agriculture, Food and Rural Affairs, Guelph ON N1G 4Y2, E-mail: [email protected] 2 – Southern Crop Protection and Food Research Centre – AAFC, London ON N5V 4T3 3 – Dept. Environmental Biology, University of Guelph, Guelph ON N1G 2W1 INTRODUCTION RESULTS Onion thrips (OT) are a cosmopolitan species that are found in onion growing 2001 – Foliar Treatments 140 120 regions around the world. They have been recorded as economic pests on onion, 120 100 100 garlic, leeks, tomatoes, cucumbers and beans. 80 80 * 60 60 * Adults and nymphs feed by piercing and sucking leaf tissues, removing cell Mean # OT/Plant 40 40 * 20 OT/Plant # Mean 20 contents (Fig. 1). Light feeding results in silvery streaks while heavy feeding may 0 55 0 Days After Treatment 23 result in plant die-back. Research has shown that onion losses can exceed 40% Days After Treatment Novaluron Spinosad (L) + B. bassiani Spinosad (H)+ B. bassiani B. bassiani Novaluron Spinosad (L) Spinosad (H) when high OT populations cannot be controlled. Spinosad (H) Lambda-cyhalothrin Control Lambda-cyhalothrin Kaolin Clay Control 2002 – Foliar Treatments 25 100 20 80 15 60 40 10 * * * * Mean # OT/Plant * * 20 Mean # OT/Plant * 5 * * * 0 * 0 275 28 Days After Treatment Days After Treatment Kaolin Lambda-cyhalothrin Novaluron (L) Lambda-cyhalothrin Spinosad (L) Spinosad (H) Control Novaluron (H) Pyriproxifen Pymetrozine Control a) b) NYSAES PHOTO c) 2003 – Foliar Treatments Fig.1.