A 22-Year Experience with Quinacrine Sterilization in a Rural Private Clinic in Midnapore, India: a Report on 5 Protocols and 1838 Cases A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WEST BENGAL STATE ELECTION COMMISSION 18, SAROJINI NAIDU SARANI (Rawdon Street) – KOLKATA 700 017 Ph No.2280-5277 ; FAX: 2280-7373 Mail ID : [email protected]
WEST BENGAL STATE ELECTION COMMISSION 18, SAROJINI NAIDU SARANI (Rawdon Street) – KOLKATA 700 017 Ph No.2280-5277 ; FAX: 2280-7373 Mail ID : [email protected] No. 1814-SEC/1D-139/2012 Kolkata, the 3rd December, 2012 ORDER In exercise of the power conferred by Sections 16 and 17 of the West Bengal Panchayat Elections Act, 2003 (West Bengal Act XXI of 2003), read with rules 26 and 27 of the West Bengal Panchayat Elections Rules, 2006, West Bengal State Election Commission, hereby publish the draft Order for delimitation of Paschim Medinipur Zilla Parishad constituencies and reservation of seats thereto. The Block(s) have been specified in column (1) of the Schedule below (hereinafter referred to as the said Schedule), the number of members to be elected to the Zilla Parishad specified in the corresponding entries in column (2), to divide the area of the Block into constituencies specified in the corresponding entries in column (3),to determine the constituency or constituencies reserved for the Scheduled Tribes (ST), Scheduled Castes (SC) or the Backward Classes (BC) specified in the corresponding entries in column (4) and the constituency or constituencies reserved for women specified in the corresponding entries in column (5) of the said schedule. The draft will be taken up for consideration by the State Election Commissioner after fifteen days from this day and any objection or suggestion with respect thereto, which may be received by the Commission within the said period, shall be duly considered. THE SCHEDULE Paschim Medinipur Zilla Parishad -

Weekly Outbreak Report
DiseaseWEEKLY Alerts/Outbreaks OUTBREAK reported and responded toREPORT by States/UTs through Integrated Disease Surveillance Program (IDSP) (3rd September to 9th September 2018) th th 36 Week District wise disease alerts/outbreaks reported in the 36 week 2018 36th Week Map Reporting Status of States/UT’s No. of States/UT’s Submitted outbreak report ( including NIL report) 22 No. of States/UT’s Submitted ‘NIL’ outbreak report 9 INTEGRATED DISEASE SURVEILLANCE PROGRAMME NATIONAL CENTRE FOR DISEASE CONTROL 22 Shamnath Marg, Civil Lines, Delhi-110054 Tel No. 23913148, Fax No. 23922677; www.idsp.nic.in 1 | P a g e 3623 th Week District wise disease alerts/outbreaks reported in the 36th week 2018 36th Week Map Disease alerts/outbreaks reported during this week (N=13) 4 3 2 2 2 2 1 1 1 1 No. of alerts/outbreaks ofNo. 0 CCHF Mumps Dengue Cholera Japanese Disease Encephalitis Food Poisoning Food Acute Acute Diarrheal Viral Hepatitis A Hepatitis Viral Disease/alerts Disease wise no of outbreaks reported under IDSP during 2015-2016 till 36th Week of Every Year Diphtheria Jaundice Enteric Fever Rubella Anthrax Mumps Acute Encephalitis… Malaria 2015 2016 2017 2018 Fever with Rash Viral Fever Disease Chikungunya Cholera Viral Hepatitis Dengue Chickenpox Measles Food Poisoning Acute Diarrheal… 0 50 100 150 200 250 300 350 400 450 500 550 600 650 700 750 800 850 900 950 1200 1400 1000 1050 1100 1150 1250 1300 1350 1450 2 | P a g e Date of Name of No. of No. of Date of Current Unique ID. Name of District Disease/ Illness Start of Comments/ Action Taken State/UT Cases Deaths Reporting Status Outbreak Cases reported from Village Capital Complex, Itanagar and Naharlagun. -

District AC No AC Name PS No Name of PS Name of BLO Official Designation Contact No Paschim Medinipur 219 Dantan 1 Sirni Primary
BLO Information District AC No AC Name PS No Name of PS Name of BLO Official Designation Contact No Paschim Medinipur 219 Dantan 1 Sirni Primary School MANIK LAL JANA AT 9064143470 Paschim Medinipur 219 Dantan 2 Sahania Sishu Siksha Kendra SHYAMALI BARMAN AT 9609083935 Paschim Medinipur 219 Dantan 3 Keshrambha High School NIRMAL KUMAR JANA GPK 8001119179 Paschim Medinipur 219 Dantan 4 Keshrambha Pry School SUCHISMITA MAITY AT 9002884832 Paschim Medinipur 219 Dantan 5 Baincha Patna Primary School SHIBKUMAR MAITY AT 7872459852 Paschim Medinipur 219 Dantan 6 Bahardaltitia Primary School KALPANA DUTTA AT 7872016922 Paschim Medinipur 219 Dantan 7 Gopinathpur Primary School SWARUP KHATUA AT 9735790286 Paschim Medinipur 219 Dantan 8 Nandakuria Primary School ADHIR KUMAR BERA Retd Teacher 8768696036 Paschim Medinipur 219 Dantan 9 Kalyanpur Primary School PHOTIK KUMAR MAITY AT 8348707979 Paschim Medinipur 219 Dantan 10 Talda SSK RINA DAS (MAITY) AT 7318670125 Paschim Medinipur 219 Dantan 11 Talda MSK BANKIM CHANDRA JANA AT 9775528022 Paschim Medinipur 219 Dantan 12 Talda Primary School SUNIL DAS AT 9609909228 Paschim Medinipur 219 Dantan 13 Solemanpur Primary School (R-1) ARCHANA DAS ICDS WORKER 8159994757 Paschim Medinipur 219 Dantan 14 Solemanpur Primary School (R-2) ABALA RANI DAS ICDS WORKER 9734549089 Paschim Medinipur 219 Dantan 15 Ramshyampur Primary School TAPAS DATTA AT 9732982644 Paschim Medinipur 219 Dantan 16 Shyamsundarpur Sishu Siksha Kendra SARATI MAJI(DAS) AT 7872016902 Paschim Medinipur 219 Dantan 17 Narayanchak Primary School -

Paschim Medinipur
AC Name of the Nodal personnel District AC Name Location of the VFCs No. of the VFC 1 Dantan-I Block Office Bivas Maity 2 Chakismilepur GP Office Padmalochan Maity 3 Mohanpur B.D.O Office Sri Prithwish Khan 4 Sautia G.P Office Sukumar Rath 5 Siyalsai G.P. Office Kesab Ghorai 6 Mohanpur G.P. Office Deepnarayan Maity 7 Nilda G.P. Office Subal Ch. Maity Paschim 8 Tanua G.P. Office Kalipada Dutta 219 Dantan Medinipur 9 Dantan-II Block Office Tapas Kumar Ray, IDO 10 Talda Gram Panchayat Office Ashutosh De, Secretary 11 Jenkapur Gram Panchayat Office Amalendu Jana, Secretary 12 Turka Gram Panchayat Office Lakshmi Kanta Sardar, EA 13 Sabra Gram Panchayat Office Santosh Kumar Das, EA 14 Sauri Kotbar Gram Panchayat Office Amit Jana, EA 15 Porolda Gram Panchayat Office Samarjit Bhunia, EA 16 Haripur Gram Panchayat Office Susanta Mandi, secretary 1 Dantan-I Block Office Ashis Kumar Dey 2 Alikosha GP Office Jhareswar Giri 3 Tararui GP Office Tapan Kumar Das 4 Manoharpur GP Office Sandip Bhunia 5 Salikotha GP Office Bijoy Krishna Das 6 Angua GP Office Saroj Kumar Patra 7 Anikola GP Office Pradip Dey 8 Dantan-I GP Office PK Rao 9 Dantan-II GP Office Manoj Banerjee Paschim 223 Keshiary 10 BDO Office, Kehiary Mukesh Panigrahi Medinipur 11 Ghritagram GP offce SUNIL DANDAPAT 12 Khajra GP Office SAMIR KUMAR MAITY 13 Santrapur GP Office MINU SHIT 14 Kusumpur GP Office SOMA MAHAPATRA 15 Baghasty GP Office PRATIK GHOSH 16 Nachipur GP Office SUSOVON PATI 17 Keshiary GP Office CHAITALI GIRI 18 Lalua GP Office CHANDAN RANA 19 Gaganeswar GP Office SOMA SAHOO 1 BDO Office Satkui Soumen Kumar Ghorai Paschim Kharagpur 2 Kharagpur SDO Office Tarun Kumar Mondal 224 Medinipur Sadar 3 Kharagpur SDO Office Amitava Das 4 Kharagpur Municipality Office Raju Paul 1 Narayangarh Block Development Office Surajit Mandal, SEO Monisankar Jana, EA 2 Mokrampur Gram Panchayat Office Biswajit Mitra, Addl. -

From Via to 1 St Tea M K G P Pingl a Eralchak Panchgeria Makarda 8 Km 1 Platoon 200/201/2 02 1 2 N D Tea M K G P Pingl a Knageri
Proposed Route March / CBM acitivty by CAPF from 13.03.2021 to 19.03.2021 Paschim Medinipur district Polling No. of Place of Route March No of Station Vulnerable Total Sections Nos to be hamlets to Distance Naka planned covered be covered planned to points Time to be under by the District be covered (BSF/CRPF) deployed Route Route Sub-Division Route march From Via To Police Station to be deployed Coy Team wise (CRPF/BSF) etc (CRPF/BSF) Date of planned March March Block/ Municipality Type of force planned Pingl 200/201/2 Eralchak Panchgeria Makarda 8 Km 1 Platoon 1 1st KGP team a 02 Pingl 202/212/2 Gobordha 1st Shift 1st knageria Barai Gourangachak 10 Km 1 Platoon 1 2nd KGP team a 13 npur,Baris Pingl Raghunathc 203/204/2 ha,Dujipu Chahat Sangar 9 Km 1 Platoon 2 1st r KGP team a hak 05 Pingl Dhaneswar Dhaneswarpur 207/208/2 Paschimbarh 10 Km 1 Platoon 2 2ndShift CompanyCRPF-A-31 2nd KGP team a pur paikan 09/210 Pingl 252/253/2 Pingla Saharda Dandasitra Kultapara 11 Km 1 Platoon 3 1st KGP team a 54 13.03.2021 Pingl 252/251/2 Gobordha PaschimMedinipur 1st Shift 1st Kunjapur Dangalsa Bardamodar 9 Km 1 Platoon 1 2nd KGP team a 52 npur,Baris Pingl Gobardhanpur 262/263/2 ha,Dujipu Dangalsa Harm 10 Km 1 Platoon 2 1st r KGP team a uttarpally 64 Pingl Gobardhan 259/260/2 Sitibinda Bar damodar 11 Km 1 Platoon 2ndShift 2nd CompanyCRPF-A-237 KGP team a pur 61 Pingl 173/174/1 Tulshichak Khanbichak Chhotokhelna 09 Km 1 Platoon 2 1st KGP team a 75/176 Pingl 180/181/1 Gobordha 1st Shift 1st Maligram Kalapunja Naya MSK 10 Km 1 Platoon 1 2nd KGP team a 82/183 -

College ID State University College Name Road City District Pin
College ID State University College name Road City District Pin Payable at IFSC No AC No MICR No BBA2-001 Bihar BBA Bihar Awadh Bihari Singh Mahavidyalaya Lalganj Vaishali Canara Bank,Lalganj Vaishali CNRB 0001252 125220150212 BBA2-003 Bihar BBA Bihar Braj Mohan Das College Dayalpur, Vaishali 844502 Allahabad Bank, Dyalpur Vaishali ALLA0210006 20263644708 844010002 BBA2-004 Bihar BBA Bihar Chandradeo Narain College Sahebganj Muzaffarpur 843125 Central Bank of India, Sahebganj Muzaffarpur CBIN0280026 2195891667 26 BBA2-005 Bihar BBA Bihar Dr S K Sinha Women's College Motihari 845401 State Bank of India, Motihari SBIN0001231 10953148213 845002003 BBA2-006 Bihar BBA Bihar Dr Ram Manohar Lohia Smarak Mahavidyalaya Muzaffarpur 842001 Canara Bank, Motijheel,Muzaffarpur CNRB0000258 0258201001285 842015002 BBA2-007 Bihar BBA Bihar Deo Chand College Hajipur, Vaishali 844101 Hajipur SBIN0012572 31505518429 844002004 BBA2-008 Bihar BBA Bihar Dr Jagannath Mishra College Muzaffarpur 842001 United bank of India, Motijheel, Muzaffarpur UTBIOMTJJ07 0825010102000 8420270002 BBA2-010 Bihar BBA Bihar Jagannath Singh College Chandauli Sitamarhi 843316 State Bank of India, Sitamarhi SBIN0004654 11621131845 843002503 BBA2-011 Bihar BBA Bihar Jamunilal Mahavidyalaya Hajipur Vaishali 844101 Punjab National Bank, Hajipur Vaishali PUNB0403700 4037000100062968 844024002 BBA2-013 Bihar BBA Bihar Jeewachh Mahavidyalaya Motipur, Muzaffarpur 843111 Punjab National Bank, Muzaffarpur PUNB0033400 0334000100194870 842024002 BBA2-015 Bihar BBA Bihar Khem Chand Tara Chand -

West Bengal Police Recruitment Board Araksha Bhavan (5Th Floor) Sector-II, Block-DJ Salt Lake City, Kolkata-700091
West Bengal Police Recruitment Board Araksha Bhavan (5th Floor) Sector-II, Block-DJ Salt Lake City, Kolkata-700091 ADVERTISEMENT NO. 01/2015/WBPRB NOTICE Recruitment to the post of Warder /Female Warder for Correctional Homes under the Department of Correctional Administration, West Bengal Applications are invited from eligible Indian citizens for the post of Warder /Female Warder for Correctional Homes under the Department of Correctional Administration, West Bengal from candidates between 18 and 27 years of age as on 01.01.2015 (relaxable for SC/ST/OBC candidates of West Bengal only and Ex-serviceman) having passed Madhyamik or equivalent examination. Willing candidates may submit their application form through on-line /off-line from 25/02/2015 to 27/03/2015. Candidates must visit the websites of West Bengal Police (policewb.gov.in) or Department of Correctional Administration (wbcorrectionalservices.gov.in) to ascertain the eligibility criteria, method of selection, scheme of examination, procedure for submission of application form and other terms & conditions. The last date of receipt of application is 27/03/2015 (upto 05:00 PM). lj!)Ytrl"itlllulll uI YY E5t lJsrrtscl Finar*e DePort*lslll Audit Branch Writcrs' Buildings, Kolkata-?00001' NOTISICATION the proviso tcj article 61-63{013.*[n exercise of the power conferred by No.tB31-F(P) dt. giroo:r, uaicd ttre ?5u' June' in parrinr *.ciiroii*. of notifioarion-N", iOg of tiie constirution of India and *ut*t the fotlowing ruies' namely:- ll?e. dre Govetlsr it h"d;;;tti io Rulu,r l.Shorttitlcarrdcommencemerrt.-(l)Tlreserulesnrlv.becal]edtheWestBengaIServices Gioup c E;r'plove;s) Rulcs' 2013' ,^peoini,r,.n;:;;;;;,i";';'J nt'*o'ption' of (2)Theyshatlcon:eintoforcewitheffeetfrori:thela!dayofHE0Bh?013' arly post or cacre or service apply in case of appoinmrent ttt z. -
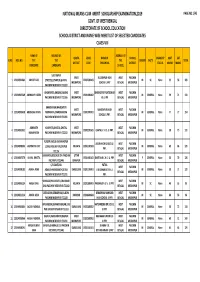
Paschim Mednipur Merit List
NATIONAL MEANS‐CUM ‐MERIT SCHOLARSHIP EXAMINATION,2019 PAGE NO.1/45 GOVT. OF WEST BENGAL DIRECTORATE OF SCHOOL EDUCATION SCHOOL DISTRICT AND NAME WISE MERIT LIST OF SELECTED CANDIDATES CLASS‐VIII NAME OF ADDRESS OF ADDRESS OF QUOTA UDISE NAME OF SCHOOL DISABILITY MAT SAT SLNO ROLL NO. THE THE THE GENDER CASTE TOTAL DISTRICT CODE THE SCHOOL DISTRICT STATUS MARKS MARKS CANDIDATE CANDIDATE SCHOOL SULTANPUR WEST SULTANPUR HIGH WEST PASCHIM 1 123194901084 ABHIJIT DAS STREET,SULTANPUR,GHATAL 19202500103 M SC None 53 56 109 MIDNAPORE SCHOOL U.PRY BENGAL MEDINIPUR PASCHIM MEDINIPUR 721222 GHONAPATA,KHELNA,SABANG WEST BHISINDIPUR YUKTESWAR WEST PASCHIM 2 123194907389 ABHRADIP HAZRA 19201000302 M GENERAL None 59 71 130 PASCHIM MEDINIPUR 721166 MIDNAPORE HS U PR BENGAL MEDINIPUR KHANDANGA,MAHESHPUR WEST MAHESHPUR HIGH WEST PASCHIM 3 123194903638 ABUBASAR KHAN MURAKATA,CHANDRAKONA 19201900701 M GENERAL None 77 77 154 MIDNAPORE SCHOOL U.PRY. BENGAL MEDINIPUR PASCHIM MEDINIPUR 721201 ADRINATH KUSHPATA,GHATAL,GHATAL WEST WEST PASCHIM 4 123194901051 19203201605 GHATAL V. H. S. U. PRY. M GENERAL None 58 73 131 MAHAPATRA PASCHIM MEDINIPUR 721212 MIDNAPORE BENGAL MEDINIPUR FULGERIA,KEUSHI,KHARAGPUR ARJUN HIGH SCHOOL U. WEST PASCHIM 5 123194908535 AGNIMITRA DEY LOCAL PASCHIM MEDINIPUR KOLKATA 19201107503 M GENERAL None 63 66 129 PRY. BENGAL MEDINIPUR 721126 SHASHINDA,BELDA,BELDA PASCHIM UTTAR WEST PASCHIM 6 123194907379 AHANA BHATTA 19201401101 DANTAN B.C.H.S. U. PRY. F GENERAL None 56 70 126 MEDINIPUR 721445 DINAJPUR BENGAL MEDINIPUR C/O‐SANTANU PATNA WEST PASCHIM 7 123194908103 AKASH ADAK ADAK,BHANGABANDH,DEBRA DARJEELING 19201100401 V.K.SIKHANIKETAN U. M GENERAL None 58 71 129 BENGAL MEDINIPUR PASCHIM MEDINIPUR 721211 PRY. -
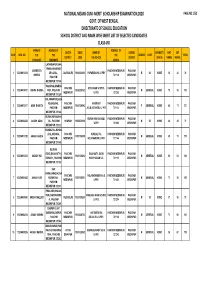
Paschim Mednipur Merit List
NATIONAL MEANS‐CUM ‐MERIT SCHOLARSHIP EXAMINATION,2020 PAGE NO.1/52 GOVT. OF WEST BENGAL DIRECTORATE OF SCHOOL EDUCATION SCHOOL DISTRICT AND NAME WISE MERIT LIST OF SELECTED CANDIDATES CLASS‐VIII NAME OF ADDRESS OF ADDRESS OF QUOTA UDISE NAME OF SCHOOL DISABILITY MAT SAT SLNO ROLL NO. THE THE THE GENDER CASTE TOTAL DISTRICT CODE THE SCHOOL DISTRICT STATUS MARKS MARKS CANDIDATE CANDIDATE SCHOOL LACHHMAPUR,KHA TRANGA,KHARAGP ABHIBRATA PASCHIM MEDINIPUR PASCHIM 1 123204913214 UR LOCAL , JALPAIGURI 19200802003 PAPARARA HS U PRY M SC NONE 50 26 76 GHORAI 721149 MEDINIPUR PASCHIM MEDINIPUR 721149 N46,SIRSA,ANANDA PASCHIM KOTA HIGH SCHOOL PASCHIM MEDINIPUR PASCHIM 2 123204919107 ABHRA BHUNIA PUR , PASCHIM 19200200102 M GENERAL NONE 75 55 130 MEDINIPUR U.PRY. 721260 MEDINIPUR MEDINIPUR 721260 BALARAMPUR,SABA NG,SABANG , PASCHIM HARIRHAT PASCHIM MEDINIPUR PASCHIM 3 123204913117 AISHI BHAKTA 19201008902 F GENERAL NONE 66 71 137 PASCHIM MEDINIPUR A.S.G.H.SCHOOL U. PRY 721144 MEDINIPUR MEDINIPUR 721144 KURAN,KURAN,GHA KURAN HIGH SCHOOL PASCHIM MEDINIPUR PASCHIM 4 123204904042 AKASH ADAK TAL , PASCHIM HOWRAH 19202500104 M SC NONE 46 33 79 U.PRY 721222 MEDINIPUR MEDINIPUR 721222 KUNDALPAL,KUNDA LPAL,SABANG , PASCHIM KUNDAL PAL PASCHIM MEDINIPUR PASCHIM 5 123204913122 AKASH HAZRA 19201008905 M GENERAL NONE 65 70 135 PASCHIM MEDINIPUR K.B.V.MANDIR U. PRY 721144 MEDINIPUR MEDINIPUR 721140 KULBANI ROAD,BAGHASTY,K PASCHIM BAGHASTY JNION PASCHIM MEDINIPUR PASCHIM 6 123204913013 AKASH HUI 19201605002 M GENERAL NONE 65 61 126 ESHIARY , PASCHIM MEDINIPUR HARICHARAN S.C. 721133 MEDINIPUR MEDINIPUR 721133 KAR PARA,KARASIA,CHA PASCHIM PALASHCHABRI N.H.S. -
Aalo Students 2013-15
Aalo Students 2013-15 Name School Home Address Percentage Subject combination of Marks in in H.S. Madhyamik 1. ARPITA KHAN Sir Ramesh Mitter Girls’ 12/H/3 Rupnarayan 83.4 Accountancy, Business (9804261485 or School Nandan Lane, Study, Economics, 8697909606) (Ph No. :) Bhowanipur, Computer Application Kolkata – 25 2. BAISHAKHI PAL Joykrishnapur High School Layer, Janta, Anchal- 86.14 Physics, Chemistry, (9564594214) (HM Ph No. : 9475121431) Radhanagar, Mathematics, Biology Bishnupur, Bankura, Pin- 722122 3. MOUMITA GIRI Contai Hindu Girls’ School Kumarpur, Contai, 73.85 Biology, Chemisty, (9002227271) (Ph No. :) Purba Medinipur, Pin - Nutrition, Computer 7221901 4. SANKAR SAMANTA Jalchak Nateswari Netaji Tilantapara, Sabang, 89.14 Physics, Chemistry, (9733746677) Vidyayatan Paschim Medinipur, Mathematics, Biology (Ph. No-03222241346 ) Pin - 7221155 5. SANDIPAN MAJHI Kharui Union High School Gujar Kharui, Jamitya, 86.3 Physics, Chemistry, (9609213508) (Ph. No.-) Tamluk, Purba Mathematics Medinipur 6. PRIYO GOPAL Bardhaman Bidyarthi Kumir Kola, Rupsa, 72.57 Physics, Chemistry, CHATTERJEE Bhaban High School Khandaghosh, Mathematics, Biology (8348052554 (Ph.No.-03422644731 ) Burdwan, Pin-713142 9593537968) 7. PALASH PAL Paramanandapur Uriasai, Garhbeta, 74.14 Physics, Chemistry, (9547480745) Jagannath Institution Paschim Medinipur, Mathematics, Biology (Ph.No: 03228236142) Pin- 721260 8. AKASH MAITI Jalchak Nateswari Netaji Kumarpur, Dumdan, 74.0 Physics, Chemistry, (8967819193) Vidyayatan Panskura, Purba Mathematics, Biology (Ph.No- 03222241346 ) Medinipur, Pin- 721152 9. RABI PATRA Contai Kshetra Mohan Pratappur, Dariapur, 74.57 Physics, Chemistry, (8768435814) Vidyabhaban Contai, Purba Mathematics, Biology (Ph. No:03220255030) Medinipur, Pin- 721442 10. RABISANKAR PAUL Rudranagar Debendra Bamankhali, Sagar, (S) 75.17 Physics, Chemistry, (7602684338 Bidyapith 24Parganas, Pin- Mathematics, Biology 9933578413) (Ph. No: 8906978341) 743373 11. -
List of Villages [Below 2000 Population] Covered by Bank Through BC Model No
List of Villages [below 2000 population] covered by Bank through BC Model No. of S No. Zone Name of State District Name of Base Branch Name of village Population Household 1 AGARTALA MIZORAM AIZAWL AIZAWL ZOHMUN 1363 235 2 AGARTALA MANIPUR BISHENPUR BISHENPUR NINGTHOUKHONG AWANG 1540 181 3 AGARTALA MANIPUR BISHENPUR BISHENPUR SUNUSHIPHAI 1388 253 4 AGARTALA MANIPUR BISHENPUR BISHENPUR YUMNUM KHUNOU 1116 188 5 AGARTALA Tripura Khowai Baganbazar Halong matai 1485 348 6 AGARTALA Tripura Khowai Baganbazar Prem Sing Orang 1127 238 7 AGARTALA Tripura North Tripura Chandrapur Abdullapur 400 67 8 AGARTALA Tripura North Tripura Chandrapur Dulakandi 900 113 9 AGARTALA Tripura North Tripura Chandrapur Durgapur 1000 125 10 AGARTALA Tripura North Tripura Chandrapur East Sakaibari 1180 135 11 AGARTALA Tripura North Tripura Chandrapur Kuterbasa 550 92 12 AGARTALA Tripura North Tripura Chandrapur Madhya Chandrapur 975 122 13 AGARTALA Tripura North Tripura Chandrapur Nathpara 900 112 14 AGARTALA Tripura North Tripura Chandrapur North Chandra Pur 950 135 15 AGARTALA Tripura North Tripura Chandrapur Radhanagar 500 72 16 AGARTALA Tripura North Tripura Chandrapur South Sakaibari 800 133 17 AGARTALA Tripura North Tripura Chandrapur West Chandrapur 1050 150 18 AGARTALA Tripura North Tripura Chandrapur West Raghna 600 86 19 AGARTALA Tripura North Tripura Chandrapur West Sakai bari 1125 142 20 AGARTALA Tripura West Tripura Mohanpur Kambukcherra 1908 317 21 AHMEDABAD GUJARAT Amreli Amreli Bhutia 1800 40 22 AHMEDABAD GUJARAT Amreli Amreli Giria 1900 30 23 AHMEDABAD -

Notice ::BLO Information
BLO Information District AC No AC Name PS No Name of PS Name of BLO Contact No Paschim Medinipur 219 Dantan 1 Sirni Primary School JADUPATI PAHARI 9609357518 Paschim Medinipur 219 Dantan 2 Sahania Sishu Siksha Kendra SHYAMALI BARMAN 9609083935 Paschim Medinipur 219 Dantan 3 Keshrambha High School NIRMAL KUMAR JANA 8001119179 Paschim Medinipur 219 Dantan 4 Keshrambha Pry School MANIKLAL JANA 8158965234 Paschim Medinipur 219 Dantan 5 Baincha Patna Primary School SHIBKUMAR MAITY 7872459852 Paschim Medinipur 219 Dantan 6 Bahardaltitia Primary School KALPANA DUTTA 7872016922 Paschim Medinipur 219 Dantan 7 Gopinathpur Primary School SWARUP KHATUA 9735790286 Paschim Medinipur 219 Dantan 8 Nandakuria Primary School ADHIR KUMAR BERA 8768696036 Paschim Medinipur 219 Dantan 9 Kalyanpur Primary School PHOTIK KUMAR MAITY 8348707979 Paschim Medinipur 219 Dantan 10 Talda SSK RINA DAS (MAITY) 7318670125 Paschim Medinipur 219 Dantan 11 Talda MSK BANKIM CHANDRA JANA 9775528022 Paschim Medinipur 219 Dantan 12 Talda Primary School SUNIL DAS 9609909228 Paschim Medinipur 219 Dantan 13 Solemanpur Primary School (R-1) SABITA ADAK 7872392191 Paschim Medinipur 219 Dantan 14 Solemanpur Primary School (R-2) ABALA RANI DAS 9734549089 Paschim Medinipur 219 Dantan 15 Ramshyampur Primary School TAPAS DATTA 9732982644 Paschim Medinipur 219 Dantan 16 Shyamsundarpur Sishu Siksha Kendra SARATI MAJI(DAS) 7872016902 Paschim Medinipur 219 Dantan 17 Narayanchak Primary School REKHA DUTTA ( GHOSH) 9635934399 Paschim Medinipur 219 Dantan 18 Srikrishnapur Primary School (R-1)