Cells from Chronic Oxidative Stress He JN1,2, Zhang SD3, Qu Y2, Wang HL2, Tham CC1, Pang CP1, Chu WK4
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Globalization of Chinese Food ANTHROPOLOGY of ASIA SERIES Series Editor: Grant Evans, University Ofhong Kong
The Globalization of Chinese Food ANTHROPOLOGY OF ASIA SERIES Series Editor: Grant Evans, University ofHong Kong Asia today is one ofthe most dynamic regions ofthe world. The previously predominant image of 'timeless peasants' has given way to the image of fast-paced business people, mass consumerism and high-rise urban conglomerations. Yet much discourse remains entrenched in the polarities of 'East vs. West', 'Tradition vs. Change'. This series hopes to provide a forum for anthropological studies which break with such polarities. It will publish titles dealing with cosmopolitanism, cultural identity, representa tions, arts and performance. The complexities of urban Asia, its elites, its political rituals, and its families will also be explored. Dangerous Blood, Refined Souls Death Rituals among the Chinese in Singapore Tong Chee Kiong Folk Art Potters ofJapan Beyond an Anthropology of Aesthetics Brian Moeran Hong Kong The Anthropology of a Chinese Metropolis Edited by Grant Evans and Maria Tam Anthropology and Colonialism in Asia and Oceania Jan van Bremen and Akitoshi Shimizu Japanese Bosses, Chinese Workers Power and Control in a Hong Kong Megastore WOng Heung wah The Legend ofthe Golden Boat Regulation, Trade and Traders in the Borderlands of Laos, Thailand, China and Burma Andrew walker Cultural Crisis and Social Memory Politics of the Past in the Thai World Edited by Shigeharu Tanabe and Charles R Keyes The Globalization of Chinese Food Edited by David Y. H. Wu and Sidney C. H. Cheung The Globalization of Chinese Food Edited by David Y. H. Wu and Sidney C. H. Cheung UNIVERSITY OF HAWAI'I PRESS HONOLULU Editorial Matter © 2002 David Y. -

Long-Term Results of Oral Valganciclovir for Treatment of Anterior Segment Inflammation Secondary to Cytomegalovirus Infection
Clinical Ophthalmology Dovepress open access to scientific and medical research Open Access Full Text Article ORIGINAL RESEARCH Long-term results of oral valganciclovir for treatment of anterior segment inflammation secondary to cytomegalovirus infection Victoria WY Wong Background: The purpose of this study was to assess the efficacy of oral valganciclovir in the Carmen KM Chan treatment of anterior segment inflammation caused by cytomegalovirus (CMV) infection. Dexter YL Leung Methods: Consecutive patients with anterior segment inflammation due to CMV causing Timothy YY Lai anterior uveitis or corneal endotheliitis treated with oral valganciclovir were reviewed. Diagnosis of CMV infection was confirmed by polymerase chain reaction of the aqueous aspirate prior Department of Ophthalmology and Visual Sciences, The Chinese to commencement of oral valganciclovir. All patients were treated with an oral loading dose University of Hong Kong, Hong Kong of 900 mg valganciclovir twice daily for at least 2 weeks, followed by an additional 450 mg Eye Hospital, Hong Kong, valganciclovir twice-daily maintenance therapy. Changes in visual acuity, intraocular pressure People’s Republic of China (IOP), use of antiglaucomatous eye drops, and recurrence were analyzed. For personal use only. Results: Thirteen eyes of 11 patients were followed for a mean of 17.2 months. Two patients had bilateral corneal endotheliitis. All eyes had absence of anterior segment inflammation within 3 weeks after treatment. Following treatment, the mean logMAR visual acuity improved significantly from 0.58 at baseline to 0.37 at the last follow-up (P = 0.048). The mean IOP and number of antiglaucomatous eye drops also decreased significantly (P = 0.021 and P = 0.004, respectively). -

Visual Acuity and Quality of Life Outcomes in Cataract Surgery Patients in Hong Kong Joseph Lau, John J Michon, Wing-Shing Chan, Leon B Ellwein
12 WORLD VIEW Br J Ophthalmol: first published as 10.1136/bjo.86.1.12 on 1 January 2002. Downloaded from Visual acuity and quality of life outcomes in cataract surgery patients in Hong Kong Joseph Lau, John J Michon, Wing-Shing Chan, Leon B Ellwein ............................................................................................................................. Br J Ophthalmol 2002;86:12–17 Series editors: Background: Visual acuity, visual functioning, and vision related quality of life outcomes after cataract W V Good and S Ruit surgery were assessed in a population based study in a suburban area of Hong Kong. Methods: A cluster sampling design was used to select apartment buildings within housing estates for enumeration. All enumerated residents 60 years of age or over were invited for an eye examination and visual acuity measurement at a site within each estate. Visual functioning (VF) and vision related quality of life (QOL) questionnaires were administered to interview subjects who had undergone cata- ract surgery and to unoperated people with presenting visual acuity less than 6/60 in either eye, and a sample of those with normal visual acuity. Results: 36.6% of the 310 cataract operated individuals had presenting visual acuity 6/18 or better in both eyes, and 40.0% when measured by pinhole. 4.5% were blind, with presenting visual acuity less than 6/60 in both eyes. Of operated eyes, 59.6% presented with visual acuity 6/18 or better. 11.2% of the operated eyes were blind with vision less than 6/60. Visual acuity outcomes 6/18 or better were marginally associated with surgery in private versus public hospitals. -

Alcohol-Assisted Epithelial Debridement for Treatment Of
ORIGINAL ARTICLE Alcohol-assisted epithelial debridement for treatment of recurrent corneal erosion: a case series Leslie KL Cheng1,2, MSc MBChB MRCSEd(Ophth), Vanissa WS Chow1,2, MBBS FRCSEd, FCOphthHK, Brian MK Yiu3, FRCSEd, FHKAM(Ophthalmology) 1Hong Kong Eye Hospital 2Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong 3Brian Yiu and Partners Ltd, Hong Kong Eye Practice Correspondence and reprint requests: Dr Leslie Cheng, Hong Kong Eye Hospital, 147K Argyle Street, Kowloon, Hong Kong SAR, China. Email: [email protected] Conclusion: Alcohol-assisted epithelial debridement is Abstract simple, safe, and effective for the treatment of recurrent corneal erosion. It can be performed in a clinic setting Purpose: Recurrent corneal erosion is characterized under slit lamp. by recurrent episodes of spontaneous breakdown of the corneal epithelium. Alcohol delamination of the corneal epithelium has a high success rate, but the procedure requires the use of an operating microscope in an Key words: Alcohols; Corneal ulcer; Epithelium, corneal operating theater. We present a case series of alcohol- assisted epithelial debridement in the clinic setting. Introduction Methods: Records of eight consecutive patients aged >18 years who presented to a private ophthalmology Recurrent corneal erosion (RCE) is characterized by practice clinic in Hong Kong between October 2012 the failure of epithelial cells to re-adhere tightly to the and March 2018 with recurrent corneal erosion that underlying stroma following injury,1 owing to a combination did not respond well to conservative or surgical of hemidesmosome weakness, pathological changes to management with persistent symptoms of >3 months the basement membrane, and excessive activity of matrix were retrospectively reviewed. -
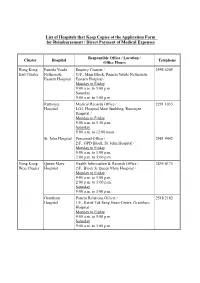
List of Hospitals That Keep Copies of the Application Form for Reimbursement / Direct Payment of Medical Expenses
List of Hospitals that Keep Copies of the Application Form for Reimbursement / Direct Payment of Medical Expenses Responsible Office / Location / Cluster Hospital Telephone Office Hours Hong Kong Pamela Youde Enquiry Counter / 2595 6205 East Cluster Nethersole G/F., Main Block, Pamela Youde Nethersole Eastern Hospital Eastern Hospital / Monday to Friday 9:00 a.m. to 5:00 p.m. Saturday 9:00 a.m. to 1:00 p.m. Ruttonjee Medical Records Office / 2291 1035 Hospital LG1, Hospital Main Building, Ruttonjee Hospital / Monday to Friday 9:00 a.m. to 5:30 p.m. Saturday 9:00 a.m. to 12:00 noon St. John Hospital Personnel Office / 2981 9442 2/F., OPD Block, St. John Hospital / Monday to Friday 9:00 a.m. to 1:00 p.m. 2:00 p.m. to 5:00 p.m. Hong Kong Queen Mary Health Information & Records Office / 2855 4175 West Cluster Hospital 2/F., Block S, Queen Mary Hospital / Monday to Friday 9:00 a.m. to 1:00 p.m. 2:00 p.m. to 5:00 p.m. Saturday 9:00 a.m. to 1:00 p.m. Grantham Patient Relations Officer / 2518 2182 Hospital 1/F., Kwok Tak Seng Heart Centre, Grantham Hospital / Monday to Friday 9:00 a.m. to 5:00 p.m. Saturday 9:00 a.m. to 1:00 p.m. - 2 - Responsible Office / Location / Cluster Hospital Telephone Office Hours Kowloon Kwong Wah Medical Report Office / 3517 5216 West Cluster Hospital 1/F., Central Stack, Kwong Wah Hospital / Monday to Friday 9:00 a.m. -

Experience from Hong Kong Eye Hospital
Int J Ophthalmol, Vol. 13, No. 6, Jun.18, 2020 www.ijo.cn Tel: 8629-82245172 8629-82210956 Email: [email protected] ·COVID-19 and Ophthalmology· Ophthalmology in the time of COVID-19: experience from Hong Kong Eye Hospital Stephanie S.L. Cheung1,2, Cherie Y.K. Wong1,2, Jason C.K. Chan1,2, Carmen K.M. Chan1,2, N.M. Lam1,2, Hunter K.L. Yuen1,2, Victoria W.Y. Wong1,2, Chi Wai Tsang1,2, Clement C.Y. Tham1,2 1Department of Ophthalmology and Visual Sciences, the ● CONCLUSION: Our multi-pronged approach to infection Chinese University of Hong Kong, Hong Kong SAR, China control is, so far, successful in minimizing infection risks, 2Hong Kong Eye Hospital, Hong Kong SAR, China while allowing the maintenance of essential ophthalmic Co-first authors: Stephanie S.L. Cheung and Cherie Y.K. services. Wong ● KEYWORDS: COVID-19; coronavirus; SARS-CoV-2; Correspondence to: Clement C.Y. Tham. Department of ophthalmology; infection control; Hong Kong Ophthalmology and Visual Sciences, the Chinese University of DOI:10.18240/ijo.2020.06.01 Hong Kong, Hong Kong SAR, China. [email protected] Received: 2020-04-09 Accepted: 2020-04-21 Citation: Cheung SSL, Wong CYK, Chan JCK, Chan CK, Lam NM, Yuen HK, Wong VW, Tsang CW, Tham CCY. Ophthalmology in the Abstract time of COVID-19: experience from Hong Kong Eye Hospital. Int J ● AIM: To review international guidelines and to share our Ophthalmol 2020;13(6):851-859 infection control experience during the coronavirus disease 2019 (COVID-19) pandemic at a tertiary eye centre in Hong Kong. -

List of Medical Social Services Units Under Social Welfare Department
List of Medical Social Services Units Under Social Welfare Department Hong Kong Name of Hospital/Clinic Tel. No. Email Address 1. Queen Mary Hospital 2255 3762 [email protected] 2255 3764 2. Wong Chuk Hang Hospital 2873 7201 [email protected] 3. Pamela Youde Nethersole Eastern 2595 6262 [email protected] Hospital 4. Pamela Youde Nethersole Eastern 2595 6773 [email protected] Hospital (Psychiatric Department) Kowloon Name of Hospital/Clinic Tel. No. Email Address 5. Tseung Kwan O Hospital 2208 0335 [email protected] 2208 0327 6. United Christian Hospital 3949 5178 [email protected] (Psychiatry) 7. Queen Elizabeth Hospital 3506 7021 [email protected] 3506 7027 3506 5499 3506 4021 8. Hong Kong Eye Hospital 2762 3069 [email protected] 9. Kowloon Hospital Rehabilitation 3129 7857 [email protected] Building 10. Kowloon Hospital 3129 6193 [email protected] 11. Kowloon Hospital 2768 8534 [email protected] (Psychiatric Department) 1 The New Territories Name of Hospital/Clinic Tel. No. Email Address 12. Prince of Wales Hospital 3505 2400 [email protected] 13. Shatin Hospital 3919 7521 [email protected] 14. Tai Po Hospital 2607 6304 [email protected] Sub-office Tai Po Hospital (Child and Adolescent 2689 2486 [email protected] Mental Health Centre) 15. North District Hospital 2683 7750 [email protected] 16. Tin Shui Wai Hospital 3513 5391 [email protected] 17. Castle Peak Hospital 2456 7401 [email protected] 18. Siu Lam Hospital 2456 7186 [email protected] 19. -

Hospital Authority's Planned Projects for 2021-2022
LC Paper No. CB(4)503/20-21(02) Head 708 Subhead 8083MM One-Off Grant to the Hospital Authority for Minor Works Projects 2021-22 Planned Projects Prepared by the Hospital Authority February 2021 Head 708 : Subhead 8083MM One-off Grant to the Hospital Authority for Minor Works Projects for the 2019-20 Financial Year Part A - Previously approved items and other items to commence in 2020-21 with expected expenditure in 2020-21 and/or 2021-22 Actual Approved Cumulative Revised Estimated cash flow in subsequent years Expenditure Estimate Priority / Project Expenditure Estimate Project Title (1.4.2020 to 2021-22 Post Item No. Estimate to 31.3.2020 2020-21 31.10.2020) 2022-23 2023-24 2024-25 2024-25 ($'000) (I) Previously approved items (up to 31.10.2020) with expected expenditure in 2020-21 and/or 2021-22 EMR15-604 Modernisation of lifts in Day Treatment Block and Special Block in Prince of Wales Hospital 16,794 16,540 254 254 - - - - - EMR16-104 Replacement of the local central control and monitoring system for Wong Chuk Hang Hospital 1,280 1,150 39 101 - 30 - - - EMR16-401 Replacement of fire alarm and detection system at Hospital Main Block in Tseung Kwan O 6,500 6,500 (1,371) (1,371) - - - - - Hospital EMR16-504 Replacement of 1 no. main switch board for Block A in Yan Chai Hospital 2,345 2,202 142 142 - - - - - EMR16-505 Replacement of building management system at Multi Services Complex in Yan Chai Hospital 3,500 3,148 55 55 297 - - - - EMR16-506 Replacement of the air handling unit for Department of Central Supporting Services at 1/F, 502 526 (24) (24) - - - - - Block B in Yan Chai Hospital EMR17-102 Replacement of emergency generators for Hospital Block at St. -

Lowerintermediates1#1 Concertsinhongkong
LESSON NOTES Lower Intermediate S1 #1 Concerts in Hong Kong CONTENTS 2 Traditional Chinese 2 Jyutping 2 English 3 Vocabulary 3 Sample Sentences 4 Grammar 5 Cultural Insight # 1 COPYRIGHT © 2013 INNOVATIVE LANGUAGE LEARNING. ALL RIGHTS RESERVED. TRADITIONAL CHINESE 1. A: 2. B: 3. A: 4. B: 5. A: 6. B: JYUTPING 1. A: go3 jam1 ngok6 wui5 zan1 hai6 hou2 zeng3! 2. B: hai6 lo1 , ngo5 hou2 zung1 ji3 mei5 gwok3 jam1 ngok6. 3. A: mei5 gwok3 ? ni1 deoi6 hai6 jing1 gwok3 ngok6 deoi2. 4. B: hai6 me1? 5. A: nei5 teng1 ng4 dou2 di1 hau2 jam1 ge3 me1? 6. B: ngo5 gok3 dak1 dou1 hai6 jat1 joeng6. ENGLISH CONT'D OVER CANTONES ECLAS S 101.COM LOWER I NTERMEDIATE S 1 #1 - CONCERTS I N HONG KONG 2 1. A: What a great concert! 2. B: Yeah, I love American music. 3. A: American? This is a British band. 4. B: Really? 5. A: Can't you hear the accent? 6. B: They're all the same to me. VOCABULARY Tr aditional Romanization English Class go1 ci4 lyrics noun ngok6 deoi2 band noun hau2 jam1 accent noun jin2 coeng3 wui5 concert noun jam1ngok6 music noun go1 sau2 singer noun concert; symphony; jam1 ngok6 wui5 recital noun go1 kuk1 song noun SAMPLE SENTENCES CANTONES ECLAS S 101.COM LOWER I NTERMEDIATE S 1 #1 - CONCERTS I N HONG KONG 3 beyond ni1 sau2 go1 ge3 go1 ci4 hou2 zuk1 dung6 jan4 heong1 gong2 zeoi3 ceot1 meng2 ge3 ngok6 sum1. deoi2 mok6 gwo3 jyu1 BEYOND. This song has very touching lyrics. -

Servant Leadership (LEAT1341)
– Servant Leadership (LEAT1341) Introduction What is servant leadership? What are the qualities/traits of a servant leader? Why should we be one? Programme Type Leadership Talk Speaker Awardee of the Hong Kong Humanity Award 2014 Awardee of 2013 The Hong Kong Youth Service Award Winner of The Fifth Hong Kong Volunteer Award Honorary Clinical Assistant Professor at Faculty of Medicine of the Chinese University of Hong Kong Associate Ophthalmologist Consultant at The Hong Kong Eye Hospital Chairman of the Public Education Committee of The Hong Kong Federation of Societies for the Prevention of Blindness Vice Chairman (External) of The Hong Kong Regeneration Society Director of Public Education & Executive Committee, Project Vision Volunteer Ophthalmologist of Project Vision, Orbis and Lifeline Express Dr. Li Yuen-mei, Emmy has been a committed volunteer since her days at secondary school. Now as a doctor, she continues her voluntary works believing that she has the responsibility to give back to the society. Dr Li works with the Hong Kong Eye Hospital and has been joining voluntary trips to serve patients in deprived areas of many provinces in the Mainland. In 2009, she helped coordinate and run the Hainan Project for Project Vision. Within a year, with the help of her teammates, some 30,000 eye surgeries were conducted, more than 10,000 eye check-ups was done and over 10 eye doctors there were trained. Remotely from Hong Kong, she offers voluntary professional advices to the Mainland ophthalmological trainees and assesses their performances through surgery video-records making sure they provide safe and high standards treatments to patients. -
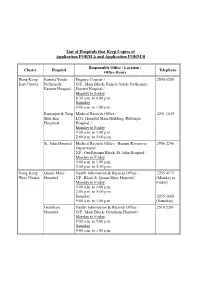
Reimbursement Forms in Designated Site
List of Hospitals that Keep Copies of Application FORM A and Application FORM B Responsible Office / Location / Cluster Hospital Telephone Office Hours Hong Kong Pamela Youde Enquiry Counter / 2595 6205 East Cluster Nethersole G/F., Main Block, Pamela Youde Nethersole Eastern Hospital Eastern Hospital / Monday to Friday 8:30 a.m. to 6:00 p.m. Saturday 9:00 a.m. to 1:00 p.m. Ruttonjee & Tang Medical Records Office / 2291 1035 Shiu Kin LG1, Hospital Main Building, Ruttonjee Hospitals Hospital / Monday to Friday 9:00 a.m. to 1:00 p.m. 2:00 p.m. to 5:00 p.m. St. John Hospital Medical Records Office / Human Resources 2986 2246 Department/ 2/F., Out-Patients Block, St. John Hospital / Monday to Friday 9:00 a.m. to 1:00 p.m. 2:00 p.m. to 5:30 p.m. Hong Kong Queen Mary Health Information & Records Office / 2255 4175 West Cluster Hospital 2/F., Block S, Queen Mary Hospital / (Monday to Monday to Friday Friday) 9:00 a.m. to 1:00 p.m. 2:00 p.m. to 5:00 p.m. Saturday 2255 3660 9:00 a.m. to 1:00 p.m. (Saturday) Grantham Health Information & Records Office / 2518 2201 Hospital G/F., Main Block, Grantham Hospital / Monday to Friday 9:00 a.m. to 5:00 p.m. Saturday 9:00 a.m. to 1:00 p.m. - 2 - Responsible Office / Location / Cluster Hospital Telephone Office Hours Kowloon Kwong Wah Medical Report Office / 3517 5216 West Cluster Hospital 12/F., Central Stack, Kwong Wah Hospital / Monday to Friday 8:45 a.m. -
(14 January 2020) List of Hospitals/Clinics Under the Hospital Authority
Social Welfare Department List of Medical Social Services Units (14 January 2020) List of Hospitals/Clinics under the Hospital Authority Hong Kong Name of Hospital/Clinic Address Tel. No. Fax. No. Opening Hours 1. Queen Mary Hospital J122, 1/F, Block J, 2255 3762 2872 8565 Mon – Fri: 8:45 am – 5:15 pm Queen Mary Hospital, 2255 3764 Lunch break: 1:00 pm to 2:00 pm Pokfulam Road, Hong Kong Sat: 9:00 am – 12:00 noon 2. Wong Chuk Hang Hospital G/F, Wong Chuk Hang Hospital, 2873 7201 2554 7318 Mon – Fri: 8:45 am – 5:15 pm 2 Wong Chuk Hang Path, Lunch break: 12:30 pm to 1:30 pm Wong Chuk Hang, Hong Kong Sat: 9:00 am – 12:00 noon 3. Western Psychiatric Centre G/F, South Wing, David Trench 2517 8141 2559 9464 Mon – Fri: 8:30 am – 6:00 pm Rehabilitation Centre, Lunch break: 1:00 pm to 2:00 pm 1F High Street, Hong Kong 4. Pamela Youde Nethersole Eastern Room 081, 1/F, Main Block, Pamela 2595 6262 2558 6023 Mon – Fri:8:45 am – 5:15 pm Hospital Youde Nethersole Eastern Hospital, Lunch break: 1:00 pm to 2:00 pm 3 Lok Man Road, Chai Wan, Sat: 9:00 am – 1:00 pm Hong Kong 5. Pamela Youde Nethersole Eastern 7/F, East Block, Pamela Youde 2595 6773 2557 4231 Mon – Fri: 8:45 am – 5:15 pm Hospital (Psychiatric Department) Nethersole Eastern Hospital, Lunch break: 1:00 pm to 2:00 pm 3 Lok Man Road, Sat: 9:00 am – 1:00 pm Chai Wan, Hong Kong Kowloon Name of Hospital/Clinic Address Tel.