Anatomical Changes with Needle Length Are Correlated with Leaf Structural and Physiological Traits Across Five Pinus Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic Diversity and Conservation of Picea Chihuahuana Martínez: a Review
Vol. 13(28), pp. 2786-2795, 9 July, 2014 DOI: 10.5897/AJB2014.13645 Article Number: CADB48845877 ISSN 1684-5315 African Journal of Biotechnology Copyright © 2014 Author(s) retain the copyright of this article http://www.academicjournals.org/AJB Review Genetic diversity and conservation of Picea chihuahuana Martínez: A review Quiñones-Pérez, Carmen Zulema1, Sáenz-Romero, Cuauhtémoc2 and Wehenkel, Christian1* 1Institute of Forestry and Wood Industry, Universidad Juárez del Estado de Durango, Durango, México. 2Institute of Agricultural and Forestry Research, Universidad Michoacana de San Nicolás de Hidalgo, Michoacán, México. Received 20 January, 2014; Accepted 16 June, 2014 The conservation of genetic diversity in tree populations is an essential component of sustainable forest management. Picea chihuahuana Martínez is an endemic conifer species in Mexico and is considered to be endangered. P. chihuahuana covers a total area of no more than 300 ha at the Sierra Madre Occidental, a mountain range that harbor a high diversity of tree species. There are 40 populations of the species that have been identified in the region, and it cannot be found elsewhere. These populations form clusters within gallery forests and are usually associated with eight other tree genera. The P. chihuahuana community is mostly well preserved. Owing to its remarkable characteristics and high conservation value, P. chihuahuana has been the subject of several studies aimed at learning more about the genetic structure, ecology and potential effects of climate change. However, the overall applicability of such studies is to confirm a dataset to develop management tools to help decision makers and to implement preservation and conservation strategies using genetic diversity. -

Propuesta De Conservación De Tres Especies Mexicanas De Picea En Peligro De Extinción
Ensayo Científico Rev. Fitotec. Mex. Vol. 38 (3): 235 - 247, 2015 PROPUESTA DE CONSERVACIÓN DE TRES ESPECIES MEXICANAS DE PICEA EN PELIGRO DE EXTINCIÓN PROPOSAL FOR CONSERVATION OF THREE ENDANGERED SPECIES OF MEXICAN SPRUCE Eduardo Mendoza-Maya1, Judith Espino-Espino2, Carmen Z. Quiñones-Pérez3, Celestino Flores-López4, Christian Wehenkel3, J. Jesús Vargas-Hernández5 y Cuauhtémoc Sáenz-Romero1* 1Instituto de Investigaciones Agropecuarias y Forestales, Universidad Michoacana de San Nicolás de Hidalgo (IIAF-UMSNH). Av. San Juanito Itzícuaro s/n. 58330, Col. San Juanito Itzícuaro. Morelia, Michoacán. 2Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo, Ciudad Universitaria Edificio B4. 58030, Col. Felícitas del Río. Morelia, Michoacán.3Instituto de Silvicultura e Industria de la Madera. Universidad Juárez del Estado de Durango. Km 5.5 carretera Durango-Mazatlán. 34120, Durango, Durango. 4Departamento Forestal, Universidad Autónoma Agraria Antonio Narro. 25000, Buenavista, Saltillo, Coahuila. 5Programa Forestal, Colegio de Postgraduados. Km 36.5 Carr. México-Texcoco. 56230, Montecillo, Texcoco, Estado de México. *Autor para correspondencia: ([email protected]) RESUMEN cana, the only four of P. martinezii and eight designed as priority of the 40 populations of P. chihuahuana, by planting individuals originated of Picea mexicana Martínez, P. chihuahuana Martínez y P. martinezii seed collected in different populations, aiming to achieve a genetically Patterson son especies endémicas de México en peligro de extinción. -

Rare and Endangered
AMERICAN CONIFER SOCIETY coniferQUARTERLY PAGE 13 Rare and Endangered SAVE THE DATES: The American Conifer Society National Meeting June 14 - 17, 2018 Summer 2017 Volume 34, Number 3 CONIFERQUARTERLY (ISSN 8755-0490) is published quarterly by the American Conifer Society. The Society is a non-profit organization incorporated under the laws of the CONIFER Commonwealth of Pennsylvania and is tax exempt under section 501(c)3 of the Internal Revenue Service Code. QUARTERLY You are invited to join our Society. Please address Editor membership and other inquiries to the American Conifer Society National Office, PO Box 1583, Minneapolis, MN Ronald J. Elardo 55311, [email protected]. Membership: US & Canada $40, International $58 (indiv.), $30 (institutional), $75 Technical Editors (sustaining), $100 (corporate business) and $150 (patron). Steven Courtney If you are moving, please notify the National Office 4 weeks David Olszyk in advance. All editorial and advertising matters should be sent to: Advisory Committee Ron Elardo, 5749 Hunter Ct., Adrian, MI 49221-2471, Tom Neff, Committee Chair (517) 902-7230 or email [email protected] Sara Malone Martin Stone Copyright © 2017, American Conifer Society. All rights reserved. No material contained herein may be reproduced Ronald J. Elardo in any form without prior written permission of the publisher. Evelyn Cox, past Editor Opinions expressed by authors and advertisers are not necessarily those of the Society. Cover Photo Keteleria davidiana Taiwan and SE Note: Hardiness Zone references in CONIFERQUARTERLY are USDA classifications unless otherwise specified. Asia. Photo by Tom Cox. Climate Zone Cwa TABLE OF CONTENTS Florida’s BIG Bald Cypress 4 FROM ASHES to REBIRTH By Ronald J. -

This Report Was Prepared As Part the North American Contribution for the State of the World's Forest Genetic Resources
This report was prepared as part the North American contribution for The State of the World’s Forest Genetic Resources (Commission on Genetic Resources for Food and Agriculture, Food and Agriculture Organization of the United Nations, Rome, 2014 available at: http://www.fao.org/forestry/fgr/64582/en/ and on the CONFORGEN website). This North American report was prepared by: Tannis Beardmore, Natural Resources Canada, Canadian Forest Service – Atlantic Region, Hugh John Fleming Forestry Centre, 1350 Regent St. S. PO 4000, Fredericton, New Brunswick, E3G 5P7. E-mail: [email protected] José Jesús Vargas Hernández, Graduate School of Forest Sciences, Colegio de Postgraduados, Km. 36.5 Carr. México-Texcoco, Montecillo, Edo. de México 56230, Mexico. Randy Johnson, National Program Leader Genetics and Global Change Research 1601 N Kent St., RPC-4, Arlington, Virginia, United States¸ 22209. Javier López-Upton, Graduate School of Forest Sciences, Colegio de Postgraduados, Km. 36.5 Carr. México-Texcoco, Montecillo, Edo. de México 56230, Mexico. Martin Williams, Natural Resources Canada, Canadian Forest Service – Atlantic Region, Hugh John Fleming Forestry Centre, 1350 Regent St. S. PO 4000, Fredericton, New Brunswick, E3G 5P7. E-mail: [email protected] 1 | P a g e North America: Regional Synthesis on the State of the World’s Forest Genetic Resources PART 1 - Regional factsheet: 1.1 Importance of forests to the region’s economy, food security, and climate change adaptation 1.1.1 Regional context North America is the third largest continent, covering 24,346,000 km2 (Food and Agriculture Organisation (FAO), 2011) and consisting of three countries: Canada, Mexico, and the United States of America (USA). -
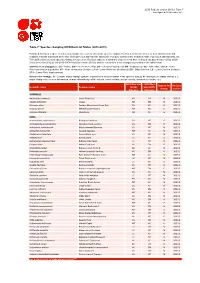
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

Gabriel Eduardo Cervantes Angel Ingeniero Forestal
UNIVERSIDAD AUTÓNOMA AGRARIA ANTONIO NARRO DIVISIÓN DE AGRONOMÍA DEPARTAMENTO FORESTAL Crecimiento de Pináceas Asociadas a Poblaciones Naturales de Picea mexicana Martínez en México Por: GABRIEL EDUARDO CERVANTES ANGEL TESIS Presentada como requisito parcial para obtener el título de: INGENIERO FORESTAL Saltillo, Coahuila, México. Diciembre, 2014 Este proyecto de tesis ha sido apoyado por el Proyecto de Investigación de la Universidad Autónoma Agraria Antonio Narro con No. 38-111-3613-2122, a cargo del profesor investigador Dr. Celestino Flores López. DEDICATORIA A Dios por ser un amigo y compañero, el motivo y guía en mi vida, todo fue posible gracias a su infinito amor, siempre te alabaré. A mis padres: Sra. Irma Angel Clemente Sr. Marcos Cervantes Molina. Quienes me dieron la vida, y procuraron darme alegría desde mi niñez. A ellos les agradezco la persona que hicieron de mí. A mi madre, por su incondicional apoyo en los momentos difíciles y por sus consejos oportunos. A mi padre, por sus regaños, consejos y la paciencia para enseñarme. Son mi orgullo, y siempre estaré agradecido con Dios por ser ustedes mis padres. Son la fortaleza y alegría de mi hogar. A mis hermanos y hermana: Sr. Eddy Ulises Cervantes Angel Sr. Marco Antonio Cervantes Angel Lic. Maricela Cervantes Angel. Quienes siempre han sido mis mejores amigos, en ustedes siempre encontré apoyo incondicional en los momentos difíciles de mi carrera y por su confianza que depositaron en mí. Son parte fundamental de mi vida y son parte de mi logro. A mi novia: Ing. Yocellyn Vázquez Ibarra. Por ser parte de mí vida, ser una persona comprensiva y apoyarme desde el inicio de nuestra hermosa relación, siempre tendrás un espacio muy especial en mi vida. -

Tree Diversity and Structure in an Abies Vejarii Martínez at the South of Nuevo León State
Revista Mexicana de Ciencias Forestales Vol. 9 (45) https://doi.org/10.29298/rmcf.v9i45.142 Article Tree diversity and structure in an Abies vejarii Martínez at the south of Nuevo León state Rigoberto González Cubas1 Eduardo Javier Treviño Garza1* Marco Aurelio González Tagle1 Álvaro Duque Montoya2 Martín Gómez Cárdenas3 1Facultad de Ciencias Forestales, Universidad de Nuevo León. México. 2Facultad de Ciencias Agrarias, Departamento de Ciencias Forestales, Universidad Nacional de Colombia-Sede Medellín. Colombia. 3Campo Experimental Uruapan. CIR-Pacífico Centro. INIFAP. México. *Autor por correspondencia, correo-e: [email protected] Revista Mexicana de Ciencias Forestales Vol. 9 (45) Abstract: To evaluate species diversity and tree density in natural forests of Abies vejarii in south Nuevo León, a directed sampling was applied in four Permanent Forest Research Sites of 2 500 m2, at 100 m high between each community. Tree diameter information was recorded for normal diameters greater than 7.5 cm. Species composition, diversity, density of individuals, basal area and dominance by species between altitudinal gradient were determined. A statistical analysis was performed with the hypothesis of equality of medians with Kruskal-Wallis nonparametric test; the effect of distance at altitude was evaluated by means of species rarefaction curves and tablecloth tests. It was a total of 553 individuals of 7 families, 10 genera and 15 species. Pinaceae and Fagaceae reached the highest importance value index; the first contributed with most species. The average forest density was 523 ha-1 individuals with an average basimetric area of 30.44 m2ha-1. The Shannon- Weiner index (H ') varied from 1.47 to 1.76. -

Three Species Endangered by Climatic Variation
1 Type of the Paper (Article, Review, Communication, etc.) 2 Provenance trials of the Mexican spruces in nursery 3 conditions: Three species endangered by climatic 4 variation 5 José Marcos Torres-Valverde1, José Ciro Hernández-Díaz2, Artemio Carrillo-Parra2, Eduardo 6 Mendoza-Maya3, Christian Wehenkel2* 7 1Maestria Institucional en Ciencias Agropecuarias y Forestales, Universidad Juárez del Estado de Durango, 8 Constitución 404 sur. Zona centro. C.P. 34000. Durango, Dgo. México. E-mail: [email protected] 9 2Instituto de Silvicultura e Industria de la Madera. Universidad Juárez del Estado de Durango, Constitución 404 10 sur. Zona centro. C.P. 34000. Durango, Dgo. México. E-mail: [email protected], [email protected] and 11 [email protected] 12 3Programa Institucional de Doctorado en Ciencias Agropecuarias y Forestales, Universidad Juárez del Estado 13 de Durango, Constitución 404 sur. Zona centro. C.P. 34000. Durango, Dgo. México. E-mail: 14 [email protected] 15 16 17 *Correspondence: *[email protected]; Tel.: 618-102-08-73 18 Abstract: The three Mexican spruces’ distributions are fragmented, which could lead to 19 phenological, morphological and genetic differentiations, partially caused by local adaptation. In 20 this study we examined the effect that climatic variables have on the survival and growth of 5,641 21 Picea seedlings, coming from eight seed provenances and produced in identical nursery conditions. 22 The respective responses of each species and provenance can be considered as a proxy of the genetic 23 differentiation and adaptation of each population. A cluster analysis revealed: i) significant 24 differences in the genetic quantitative traits among the three Picea species and ii) significant 25 correlations among genetic quantitative traits and climatic factors. -

Climate Change and Conservation
DOI: 10.2478/v10303-012-0005-4 Acta Silv. Lign. Hung., Vol. 8 (2012) 57–74 Climate Change and Conservation ∗ F. Thomas LEDIG Department of Plant Sciences, University of California, Davis, California Abstract – Conserving forest genetic resources and, indeed, preventing species extinctions will be complicated by the expected changes in climate projected for the next century and beyond. This paper uses case examples from rare spruces (Picea sp.) from North America to discuss the interplay of conservation, genetics, and climate change. New models show how climate change will affect these spruces, making it necessary to relocate them if they are to survive, a tool known as assisted migration or, preferably, assisted colonization. The paper concludes with some speculation on the broader implications of climate change, and the relevance of conservation to preserving the necessary ecological services provided by forests. conservation genetics / assisted colonization / extinction / Picea mexicana / Picea martinezii / Picea chihuahuana / Picea breweriana Kivonat – Klímaváltozás és génmegőrzés. Az erdészeti genetikai erőforrások megőrzése, és különösen a fajkihalások megakadályozása az évszázad végére (és azutánra) előrevetített klímaváltozás fényében nehéz feladatnak tűnik. A tanulmány ritka észak-amerikai lucfenyő fajok példáján tárgyalja a klímaváltozásból adódó genetikai és megőrzési problémák együttesét. Modellek mutatják be klímaváltozás hatását a luc fajok elterjedésére. Ezek szerint szükség lesz a fajok megmentése érdekében a mesterséges -

Recent Evolution and Divergence Among Populations of a Rare Mexican Endemic, Chihuahua Spruce, Following Holocene Climatic Warming
Evolution, 51(6), 1997, pp. 1815-1827 RECENT EVOLUTION AND DIVERGENCE AMONG POPULATIONS OF A RARE MEXICAN ENDEMIC, CHIHUAHUA SPRUCE, FOLLOWING HOLOCENE CLIMATIC WARMING E THOMASLEDIG,~.~ VIRGINIA JACOB-CERVANTES,~ PAUL D. HODGSKISS,~ AND TEOBALDOEGUILUZ-PIEDRA~ 'Institute of Forest Genetics, PaciJic Southwest Research Station, USDA Forest Service, 2480 Carson Road, Placerville, California 95667 2E-mail: fswds =t. ledig/ou =rO5jU3d57a@rnh~.attmail.com 3Centro de Genetica Forestal, Universidad Autonoma Chapingo, Apartado Postal No. 37, Chapingo, Mixico C.P. 56230. Mtfxico Abstract.-Fragmentation and reduction in population size are expected to reduce genetic diversity. However, examples from natural populations of forest trees are scarce. The range of Chihuahua spruce retreated northward and fragmented coincident with the warming climate that marked the early Holocene. The isolated populations vary from 15 to 2441 trees, which provided an opportunity to test whether census number is a good predictor of genetic diversity. Mean expected heterozygosity, He, based on 24 loci in 16 enzyme systems, was 0.093 for 10 sampled populations, which is within the range reported for conifers. However, estimates varied more than twofold among populations and He was closely related to the logarithm of the number of mature trees in the population (rHe,N= 0.93). Diversity among populations, FST, was 24.8% of the total diversity, which is higher than that observed in almost all conifer species studied. Nei's genetic distance, D, was not related to geographic distance between populations, and was 0.033, which is higher than estimates for most wide-ranging species. Most populations had excess homozygosity and the fixation index, Frs, was higher than that reported for all but one species of conifer. -

A New Phylogeny for the Genus Picea from Plastid, Mitochondrial, and Nuclear Sequences ⇑ Jared D
Molecular Phylogenetics and Evolution 69 (2013) 717–727 Contents lists available at SciVerse ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev A new phylogeny for the genus Picea from plastid, mitochondrial, and nuclear sequences ⇑ Jared D. Lockwood a,1, Jelena M. Aleksic´ b,1, Jiabin Zou c,1, Jing Wang c, Jianquan Liu c, Susanne S. Renner a, a Systematic Botany and Mycology, University of Munich (LMU), Menzinger Strasse 67, 80638 Munich, Germany b University of Belgrade, Institute of Molecular Genetics and Genetic Engineering, 11010 Belgrade, Serbia c State Key Laboratory of Grassland Agro-ecosystem, School of Life Sciences, Lanzhou University, Lanzhou, Gansu 730000, China article info abstract Article history: Studies over the past ten years have shown that the crown groups of most conifer genera are only about Received 28 March 2013 15–25 Ma old. The genus Picea (spruces, Pinaceae), with around 35 species, appears to be no exception. In Revised 21 June 2013 addition, molecular studies of co-existing spruce species have demonstrated frequent introgression. Per- Accepted 5 July 2013 haps not surprisingly therefore previous phylogenetic studies of species relationships in Picea, based Available online 18 July 2013 mostly on plastid sequences, suffered from poor statistical support. We therefore generated mitochon- drial, nuclear, and further plastid DNA sequences from carefully sourced material, striking a balance Keywords: between alignability with outgroups and phylogenetic signal content. Motif duplications in mitochon- Molecular clocks drial introns were treated as characters in a stochastic Dollo model; molecular clock models were cali- Historical biogeography Mitochondrial nad introns brated with fossils; and ancestral ranges were inferred under maximum likelihood. -

Mexican Conifers Differ in Their Capacity to Face Climate Change C
Mexican conifers differ in their capacity to face climate change C. Sáenz-Romero, M. Larter, N. González-Muñoz, C. Wehenkel, A. Blanco-Garcia, D. Castellanos-Acuña, R Burlett, S Delzon To cite this version: C. Sáenz-Romero, M. Larter, N. González-Muñoz, C. Wehenkel, A. Blanco-Garcia, et al.. Mexican conifers differ in their capacity to face climate change. Journal of Plant Hydraulics, INRA Science and Impact, 2017, 4, pp.e003. 10.20870/jph.2017.e003. hal-01445515 HAL Id: hal-01445515 https://hal.archives-ouvertes.fr/hal-01445515 Submitted on 25 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Journal of Plant Hydraulics 4: e-003 Mexican conifers differ in their capacity to face climate change C. Sáenz-Romero1,2, M. Larter2, N. González-Muñoz2, C. Wehenkel3, A. Blanco-Garcia4, D. 1,5 2 2 Castellanos-Acuña , R. Burlett and S. Delzon 1Universidad Michoacana de San Nicolás de Hidalgo, Instituto de Investigaciones Agropecuarias y Forestales (UMSNH-IIAF). Km 9.5 Carretera Morelia-Zinapécuaro, Tarímbaro Michoacán 58880, México; 2Institut National de la Recherche Agronomique (INRA), Unité Mixte de Recherche 1202 Biodiversité Gènes & Communautés (UMR 1202 BIOGECO), F-33610 Cestas, France & Université de Bordeaux, F-33615 Pessac, France; 3Instituto de Silvicultura e Industria de la Madera, Universidad Juárez del Estado de Durango, Aptdo.