The Mating System and Genic Diversity in Martínez
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -

Picea Omorika Cultivation and Uses
Picea omorika From Wikipedia, the free encyclopedia Kingdom: Plantae Division: Pinophyta Picea omorika ( Serbian Spruce ) is a rare, local Class: Pinopsida spruce, endemic to the Drina River valley in western Serbia and eastern Bosnia and Herzegovina Order: Pinales near Višegrad. It was originally discovered near the Family: Pinaceae village of Zaovine on the Tara Mountain in 1875, č ć Genus: Picea and named by the Serbian botanist Josif Pan i ; the epithet omorika is simply the Serbian word for P. omorika Species: "spruce"; hence, the scientific name means"Spruce- spruce". It is a medium-sized evergreen tree growing to 20–35 m tall, exceptionally to 40 m tall, and with a trunk diameter of up to 1 m. The shoots are buff-brown, and densely pubescent (hairy). The leaves are needle-like, 10–20 mm long, flattened in cross-section, and dark blue-green above, and blue-white below. The cones are 4–7 cm long, fusiform (spindle-shaped, broadest in the middle), dark purple (almost black) when young, maturing dark brown 5–7 months after pollination, and have stiff scales. Cultivation and uses Outside of its native range, Serbian Spruce is of major importance in horticulture as an ornamental tree in large gardens, valued in northern Europe and North America for its very attractive crown form and ability to grow on a wide range of soils, including alkaline, clay, acid and sandy soil, although it prefers moist, drained loam. It is also grown to a small extent in forestry for christmas trees, timber and paper production, particularly in northern Europe, though its slow growth makes it less important than Sitka Spruce or Norway Spruce. -

Genetic Diversity and Conservation of Picea Chihuahuana Martínez: a Review
Vol. 13(28), pp. 2786-2795, 9 July, 2014 DOI: 10.5897/AJB2014.13645 Article Number: CADB48845877 ISSN 1684-5315 African Journal of Biotechnology Copyright © 2014 Author(s) retain the copyright of this article http://www.academicjournals.org/AJB Review Genetic diversity and conservation of Picea chihuahuana Martínez: A review Quiñones-Pérez, Carmen Zulema1, Sáenz-Romero, Cuauhtémoc2 and Wehenkel, Christian1* 1Institute of Forestry and Wood Industry, Universidad Juárez del Estado de Durango, Durango, México. 2Institute of Agricultural and Forestry Research, Universidad Michoacana de San Nicolás de Hidalgo, Michoacán, México. Received 20 January, 2014; Accepted 16 June, 2014 The conservation of genetic diversity in tree populations is an essential component of sustainable forest management. Picea chihuahuana Martínez is an endemic conifer species in Mexico and is considered to be endangered. P. chihuahuana covers a total area of no more than 300 ha at the Sierra Madre Occidental, a mountain range that harbor a high diversity of tree species. There are 40 populations of the species that have been identified in the region, and it cannot be found elsewhere. These populations form clusters within gallery forests and are usually associated with eight other tree genera. The P. chihuahuana community is mostly well preserved. Owing to its remarkable characteristics and high conservation value, P. chihuahuana has been the subject of several studies aimed at learning more about the genetic structure, ecology and potential effects of climate change. However, the overall applicability of such studies is to confirm a dataset to develop management tools to help decision makers and to implement preservation and conservation strategies using genetic diversity. -

Spatial Genetic Structure in the Very Rare and Species-Rich Picea Chihuahuana Tree Community (Mexico)
Quinones-Perez et. al.·Silvae Genetica (2014) 63-4, 149-159 SAS INSTITUTE INC. (2011): SAS SAS/STAT 9.3 Computer THOMAS, S. C. (2011): Genetic vs. phenotypic responses of Software. Cary, N C, USA. trees to altitude. Tree Physiology 31(11): 1161–1163. SCHMIDTLING, R. C. (1994): Use of provenance tests to pre- VAN ZONNEVELD, M., A. JARVIS, W. DVORAK, G. LEMA and dict response to climate change: loblolly pine and Nor- C. LEIBING (2009): Climate change impact predictions way spruce. Tree Physiology 14(7-8-9): 805–817. on Pinus patula and Pinus tecunumanii populations in SOTO-CORREA, J. C., C. SÁENZ-ROMERO, R. LINDIG-CIS- Mexico and Central America. Forest Ecology and Man- NEROS, N. M. SÁNCHEZ–VARGAS and J. CRUZ-DE-LEÓN agement 257(7): 1566–1576. (2012): Genetic variation between Lupinus elegans VIVEROS-VIVEROS, H., C. SÁENZ-ROMERO, J. L. UPTON and Kunth provenances, altitudinal seed zoning and assist- J. V. HERNÁNDEZ (2005): Variación genética altitudinal ed migration. Agrociencia 46(6): 593–608. en el crecimiento de plantas de Pinus pseudostrobus ST. CLAIR, J. B. (2006): Genetic variation in fall cold har- Lindl: en campo. Agrociencia 39(5): 575–587. diness in coastal Douglas-fir in western Oregon and WEINSTEIN, A. (1989): Provenance evaluation of Pinus Washington. Canadian Journal of Botany 84(7): halepensis, P. brutia and P. eldarica in Israel. Forest 1110–1121. Ecology and Management 26(3): 215–225. TCHEBAKOVA, N. M., G. E. REHFELDT and E. I. PARFENOVA WRIGHT, J. A., L. F. OSORIO and W. S. DVORAK (1995): (2006): Impacts of climate change on the distribution of Recent developments in a tree improvement program Larix Spp. -

Propuesta De Conservación De Tres Especies Mexicanas De Picea En Peligro De Extinción
Ensayo Científico Rev. Fitotec. Mex. Vol. 38 (3): 235 - 247, 2015 PROPUESTA DE CONSERVACIÓN DE TRES ESPECIES MEXICANAS DE PICEA EN PELIGRO DE EXTINCIÓN PROPOSAL FOR CONSERVATION OF THREE ENDANGERED SPECIES OF MEXICAN SPRUCE Eduardo Mendoza-Maya1, Judith Espino-Espino2, Carmen Z. Quiñones-Pérez3, Celestino Flores-López4, Christian Wehenkel3, J. Jesús Vargas-Hernández5 y Cuauhtémoc Sáenz-Romero1* 1Instituto de Investigaciones Agropecuarias y Forestales, Universidad Michoacana de San Nicolás de Hidalgo (IIAF-UMSNH). Av. San Juanito Itzícuaro s/n. 58330, Col. San Juanito Itzícuaro. Morelia, Michoacán. 2Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo, Ciudad Universitaria Edificio B4. 58030, Col. Felícitas del Río. Morelia, Michoacán.3Instituto de Silvicultura e Industria de la Madera. Universidad Juárez del Estado de Durango. Km 5.5 carretera Durango-Mazatlán. 34120, Durango, Durango. 4Departamento Forestal, Universidad Autónoma Agraria Antonio Narro. 25000, Buenavista, Saltillo, Coahuila. 5Programa Forestal, Colegio de Postgraduados. Km 36.5 Carr. México-Texcoco. 56230, Montecillo, Texcoco, Estado de México. *Autor para correspondencia: ([email protected]) RESUMEN cana, the only four of P. martinezii and eight designed as priority of the 40 populations of P. chihuahuana, by planting individuals originated of Picea mexicana Martínez, P. chihuahuana Martínez y P. martinezii seed collected in different populations, aiming to achieve a genetically Patterson son especies endémicas de México en peligro de extinción. -

Plant L I V E Grow
VERMONT TREE SELECTION GUIDE PLANT LIVE GROW Vermont Urban & Community Forestry Program part of the Vermont Department of Forests, Parks & Recreation in partnership with the University of Vermont Extension Table of Contents INTRODUCTION 1 SITE CONDITIONS 3 SPECIAL CONSIDERATIONS 6 TREE SELECTION WORKSHEET 8 KEY TO TREE SPECIES LIST 9 KEY TO SCIENTIFIC NAMES 10 RESOURCES FOR MORE INFORMATION 11 TREE SPECIES LIST 12 The guide was funded in part by the USDA Forest Service, State and Private Forestry. Recognition is given to all the people who offered assistance to this project, especially Pamelia Smith, professor, and Elizabeth Clark, graduate, of Vermont Technical College who helped develop the tree list, to David Schneider, Warren Spinner, and Jeff Young for their review, and to Sensible World for the design. VERMONT TREE SELECTION GUIDE Introduction Are you getting ready to plant a tree or maybe several Consider the following four questions before establishing trees? Whether you are planning to plant on your own trees for long-term growth and health: lawn, in a community park, along a street, or in a tree pit, careful tree selection is essential to the tree’s long- • What is the purpose and use of the term success. We have all heard time and time again to planting? plant ‘the right tree in the right place’. Our latest Tree • What are the site conditions above and Selection Guide for Vermont was developed just for this below ground? purpose - to help you match trees to sites to achieve • What type of maintenance will be lasting shade. required? • What is the best tree species for long- To use this guide, you should first consider four term success? questions that will help you critically evaluate the planting purpose, the site, future needs and desires. -

Rare and Endangered
AMERICAN CONIFER SOCIETY coniferQUARTERLY PAGE 13 Rare and Endangered SAVE THE DATES: The American Conifer Society National Meeting June 14 - 17, 2018 Summer 2017 Volume 34, Number 3 CONIFERQUARTERLY (ISSN 8755-0490) is published quarterly by the American Conifer Society. The Society is a non-profit organization incorporated under the laws of the CONIFER Commonwealth of Pennsylvania and is tax exempt under section 501(c)3 of the Internal Revenue Service Code. QUARTERLY You are invited to join our Society. Please address Editor membership and other inquiries to the American Conifer Society National Office, PO Box 1583, Minneapolis, MN Ronald J. Elardo 55311, [email protected]. Membership: US & Canada $40, International $58 (indiv.), $30 (institutional), $75 Technical Editors (sustaining), $100 (corporate business) and $150 (patron). Steven Courtney If you are moving, please notify the National Office 4 weeks David Olszyk in advance. All editorial and advertising matters should be sent to: Advisory Committee Ron Elardo, 5749 Hunter Ct., Adrian, MI 49221-2471, Tom Neff, Committee Chair (517) 902-7230 or email [email protected] Sara Malone Martin Stone Copyright © 2017, American Conifer Society. All rights reserved. No material contained herein may be reproduced Ronald J. Elardo in any form without prior written permission of the publisher. Evelyn Cox, past Editor Opinions expressed by authors and advertisers are not necessarily those of the Society. Cover Photo Keteleria davidiana Taiwan and SE Note: Hardiness Zone references in CONIFERQUARTERLY are USDA classifications unless otherwise specified. Asia. Photo by Tom Cox. Climate Zone Cwa TABLE OF CONTENTS Florida’s BIG Bald Cypress 4 FROM ASHES to REBIRTH By Ronald J. -

This Report Was Prepared As Part the North American Contribution for the State of the World's Forest Genetic Resources
This report was prepared as part the North American contribution for The State of the World’s Forest Genetic Resources (Commission on Genetic Resources for Food and Agriculture, Food and Agriculture Organization of the United Nations, Rome, 2014 available at: http://www.fao.org/forestry/fgr/64582/en/ and on the CONFORGEN website). This North American report was prepared by: Tannis Beardmore, Natural Resources Canada, Canadian Forest Service – Atlantic Region, Hugh John Fleming Forestry Centre, 1350 Regent St. S. PO 4000, Fredericton, New Brunswick, E3G 5P7. E-mail: [email protected] José Jesús Vargas Hernández, Graduate School of Forest Sciences, Colegio de Postgraduados, Km. 36.5 Carr. México-Texcoco, Montecillo, Edo. de México 56230, Mexico. Randy Johnson, National Program Leader Genetics and Global Change Research 1601 N Kent St., RPC-4, Arlington, Virginia, United States¸ 22209. Javier López-Upton, Graduate School of Forest Sciences, Colegio de Postgraduados, Km. 36.5 Carr. México-Texcoco, Montecillo, Edo. de México 56230, Mexico. Martin Williams, Natural Resources Canada, Canadian Forest Service – Atlantic Region, Hugh John Fleming Forestry Centre, 1350 Regent St. S. PO 4000, Fredericton, New Brunswick, E3G 5P7. E-mail: [email protected] 1 | P a g e North America: Regional Synthesis on the State of the World’s Forest Genetic Resources PART 1 - Regional factsheet: 1.1 Importance of forests to the region’s economy, food security, and climate change adaptation 1.1.1 Regional context North America is the third largest continent, covering 24,346,000 km2 (Food and Agriculture Organisation (FAO), 2011) and consisting of three countries: Canada, Mexico, and the United States of America (USA). -

2021 Plant List
2021 Plant List New items are listed with an asterisk (*) Conifers Pinus thungerbii Abies koreana 'Horstmann's Silberlocke' Pinus x 'Jane Kluis' * Chamaecyparis nootkatensis 'Pendula' Sciadopitys vert. 'Joe Dozey' Chamaecyparis noot. 'Glauca Pendula' Sciadopitys vert. 'Wintergreen' Chamaecyparis obtusa 'Chirimen' * Taxodium distichum 'Pendula' Chamaecyparis obtusa 'Gracilis' -Select Taxodium distichum 'Peve Mineret' Chamaecyparis obtusa 'Kosteri' Taxus cuspidaata 'Nana Aurescens' Chamaecyparis obtusa 'Nana' Tsuga con. 'Jervis' Chamaecyparis obtusa 'Nana Gracilis' Chamaecyparis obtusa 'Spiralis' Ferns Chamaecyparis obtusa 'Thoweil' Adiantum pedatum ….Maiden Hair Chamaecyparis obtusa 'Verdoni' Athyrum filix-femina 'Minutissima' Juniperus procumbens 'Nana' Athyrium 'Ghost' Larix decidua 'Pendula' Athyrum niponicum 'Godzilla' Larix decidua 'Pendula' -Prostrate Form Athyrum niponicum 'Pictum' Picea abies 'Hasin' * Athyrum niponicum pic. 'Pearly White' Picea abies 'Pusch' * Dennstaedtia punctilobula Picea omorika 'Nana' Dryopteris ery. 'Brilliance' Picea omorika 'Pendula' Dryopteris marginalis Picea orientalis 'Nana' Matteucciastruthiopteris var. pensylvanica Picea orientalis 'Shadow's Broom' * Osmunda cinnamomea Picea pungens 'Glauca Globosa' Polystichum acrostichoides Pinus mugo 'Mughus' - Rock Garden Strain Polystichum polyblepharum Pinus mugo 'Slowmound' Pinus nigra 'Hornibrookiana' Grasses Pinus parviflora 'Aoi' These are but a fraction of the grasses we'll be Pinus parviflora 'Glauca Nana' offering this year. Many more to come. They'll -

Master Species List for Temple Ambler Field Station
Temple Ambler Field Station master species' list Figure 1. Animal groups identified to date through our citizen science initiatives at Temple Ambler Field Station. Values represent unique taxa identified in the field to the lowest taxonomic level possible. These data were collected by field citizen scientists during events on campus or were recorded in public databases (iNaturalist and eBird). Want to become a Citizen Science Owlet too? Check out our Citizen Science webpage. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station master species' list Figure 2. Plant diversity identified to date in the natural environments and designed gardens of the Temple Ambler Field Station and Ambler Arboretum. These values represent unique taxa identified to the lowest taxonomic level possible. Highlighted are 14 of the 116 flowering plant families present that include 524 taxonomic groups. A full list can be found in our species database. Cultivated specimens in our Greenhouse were not included here. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station master species' list database_title Temple Ambler Field Station master species' list last_update 22October2020 description This database includes all species identified to their lowest taxonomic level possible in the natural environments and designed gardens on the Temple Ambler campus. These are occurrence records and each taxa is only entered once. This is an occurrence record, not an abundance record. IDs were performed by senior scientists and specialists, as well as citizen scientists visiting campus. -

The Plant List
the list A Companion to the Choosing the Right Plants Natural Lawn & Garden Guide a better way to beautiful www.savingwater.org Waterwise garden by Stacie Crooks Discover a better way to beautiful! his plant list is a new companion to Choosing the The list on the following pages contains just some of the Right Plants, one of the Natural Lawn & Garden many plants that can be happy here in the temperate Pacific T Guides produced by the Saving Water Partnership Northwest, organized by several key themes. A number of (see the back panel to request your free copy). These guides these plants are Great Plant Picks ( ) selections, chosen will help you garden in balance with nature, so you can enjoy because they are vigorous and easy to grow in Northwest a beautiful yard that’s healthy, easy to maintain and good for gardens, while offering reasonable resistance to pests and the environment. diseases, as well as other attributes. (For details about the GPP program and to find additional reference materials, When choosing plants, we often think about factors refer to Resources & Credits on page 12.) like size, shape, foliage and flower color. But the most important consideration should be whether a site provides Remember, this plant list is just a starting point. The more the conditions a specific plant needs to thrive. Soil type, information you have about your garden’s conditions and drainage, sun and shade—all affect a plant’s health and, as a particular plant’s needs before you purchase a plant, the a result, its appearance and maintenance needs. -
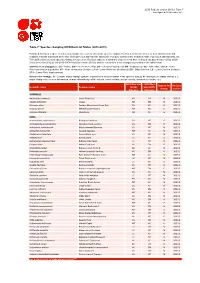
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying