Climate Change and Conservation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic Diversity and Conservation of Picea Chihuahuana Martínez: a Review
Vol. 13(28), pp. 2786-2795, 9 July, 2014 DOI: 10.5897/AJB2014.13645 Article Number: CADB48845877 ISSN 1684-5315 African Journal of Biotechnology Copyright © 2014 Author(s) retain the copyright of this article http://www.academicjournals.org/AJB Review Genetic diversity and conservation of Picea chihuahuana Martínez: A review Quiñones-Pérez, Carmen Zulema1, Sáenz-Romero, Cuauhtémoc2 and Wehenkel, Christian1* 1Institute of Forestry and Wood Industry, Universidad Juárez del Estado de Durango, Durango, México. 2Institute of Agricultural and Forestry Research, Universidad Michoacana de San Nicolás de Hidalgo, Michoacán, México. Received 20 January, 2014; Accepted 16 June, 2014 The conservation of genetic diversity in tree populations is an essential component of sustainable forest management. Picea chihuahuana Martínez is an endemic conifer species in Mexico and is considered to be endangered. P. chihuahuana covers a total area of no more than 300 ha at the Sierra Madre Occidental, a mountain range that harbor a high diversity of tree species. There are 40 populations of the species that have been identified in the region, and it cannot be found elsewhere. These populations form clusters within gallery forests and are usually associated with eight other tree genera. The P. chihuahuana community is mostly well preserved. Owing to its remarkable characteristics and high conservation value, P. chihuahuana has been the subject of several studies aimed at learning more about the genetic structure, ecology and potential effects of climate change. However, the overall applicability of such studies is to confirm a dataset to develop management tools to help decision makers and to implement preservation and conservation strategies using genetic diversity. -

Spatial Genetic Structure in the Very Rare and Species-Rich Picea Chihuahuana Tree Community (Mexico)
Quinones-Perez et. al.·Silvae Genetica (2014) 63-4, 149-159 SAS INSTITUTE INC. (2011): SAS SAS/STAT 9.3 Computer THOMAS, S. C. (2011): Genetic vs. phenotypic responses of Software. Cary, N C, USA. trees to altitude. Tree Physiology 31(11): 1161–1163. SCHMIDTLING, R. C. (1994): Use of provenance tests to pre- VAN ZONNEVELD, M., A. JARVIS, W. DVORAK, G. LEMA and dict response to climate change: loblolly pine and Nor- C. LEIBING (2009): Climate change impact predictions way spruce. Tree Physiology 14(7-8-9): 805–817. on Pinus patula and Pinus tecunumanii populations in SOTO-CORREA, J. C., C. SÁENZ-ROMERO, R. LINDIG-CIS- Mexico and Central America. Forest Ecology and Man- NEROS, N. M. SÁNCHEZ–VARGAS and J. CRUZ-DE-LEÓN agement 257(7): 1566–1576. (2012): Genetic variation between Lupinus elegans VIVEROS-VIVEROS, H., C. SÁENZ-ROMERO, J. L. UPTON and Kunth provenances, altitudinal seed zoning and assist- J. V. HERNÁNDEZ (2005): Variación genética altitudinal ed migration. Agrociencia 46(6): 593–608. en el crecimiento de plantas de Pinus pseudostrobus ST. CLAIR, J. B. (2006): Genetic variation in fall cold har- Lindl: en campo. Agrociencia 39(5): 575–587. diness in coastal Douglas-fir in western Oregon and WEINSTEIN, A. (1989): Provenance evaluation of Pinus Washington. Canadian Journal of Botany 84(7): halepensis, P. brutia and P. eldarica in Israel. Forest 1110–1121. Ecology and Management 26(3): 215–225. TCHEBAKOVA, N. M., G. E. REHFELDT and E. I. PARFENOVA WRIGHT, J. A., L. F. OSORIO and W. S. DVORAK (1995): (2006): Impacts of climate change on the distribution of Recent developments in a tree improvement program Larix Spp. -

Propuesta De Conservación De Tres Especies Mexicanas De Picea En Peligro De Extinción
Ensayo Científico Rev. Fitotec. Mex. Vol. 38 (3): 235 - 247, 2015 PROPUESTA DE CONSERVACIÓN DE TRES ESPECIES MEXICANAS DE PICEA EN PELIGRO DE EXTINCIÓN PROPOSAL FOR CONSERVATION OF THREE ENDANGERED SPECIES OF MEXICAN SPRUCE Eduardo Mendoza-Maya1, Judith Espino-Espino2, Carmen Z. Quiñones-Pérez3, Celestino Flores-López4, Christian Wehenkel3, J. Jesús Vargas-Hernández5 y Cuauhtémoc Sáenz-Romero1* 1Instituto de Investigaciones Agropecuarias y Forestales, Universidad Michoacana de San Nicolás de Hidalgo (IIAF-UMSNH). Av. San Juanito Itzícuaro s/n. 58330, Col. San Juanito Itzícuaro. Morelia, Michoacán. 2Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo, Ciudad Universitaria Edificio B4. 58030, Col. Felícitas del Río. Morelia, Michoacán.3Instituto de Silvicultura e Industria de la Madera. Universidad Juárez del Estado de Durango. Km 5.5 carretera Durango-Mazatlán. 34120, Durango, Durango. 4Departamento Forestal, Universidad Autónoma Agraria Antonio Narro. 25000, Buenavista, Saltillo, Coahuila. 5Programa Forestal, Colegio de Postgraduados. Km 36.5 Carr. México-Texcoco. 56230, Montecillo, Texcoco, Estado de México. *Autor para correspondencia: ([email protected]) RESUMEN cana, the only four of P. martinezii and eight designed as priority of the 40 populations of P. chihuahuana, by planting individuals originated of Picea mexicana Martínez, P. chihuahuana Martínez y P. martinezii seed collected in different populations, aiming to achieve a genetically Patterson son especies endémicas de México en peligro de extinción. -

ÁREAS NATURALES PROTEGIDAS DE MÉXICO Núm Región Nombre Del Área Categoría De Manejo Estados
ÁREAS NATURALES PROTEGIDAS DE MÉXICO Núm Región Nombre del Área Categoría de Manejo Estados 1 Centro y Eje Neovolcánico Barranca de Metztitlán Reserva de la Biosfera Hidalgo Estado de México y 2 Centro y Eje Neovolcánico Bosencheve Parque Nacional Michoacán 3 Centro y Eje Neovolcánico Cerro de La Estrella Parque Nacional Ciudad de México 4 Centro y Eje Neovolcánico Cerro de Las Campanas Parque Nacional Querétaro 5 Centro y Eje Neovolcánico Ciénegas del Lerma Área de Protección de Flora y Fauna Estado de México Ciudad de México, 6 Centro y Eje Neovolcánico Corredor Biológico Chichinautzin Área de Protección de Flora y Fauna Morelos y Estado de México 7 Centro y Eje Neovolcánico Cumbres del Ajusco Parque Nacional Ciudad de México 8 Centro y Eje Neovolcánico Desierto de los Leones Parque Nacional Ciudad de México 9 Centro y Eje Neovolcánico Desierto del Carmen o de Nixcongo Parque Nacional Estado de México 10 Centro y Eje Neovolcánico El Chico Parque Nacional Hidalgo 11 Centro y Eje Neovolcánico El Cimatario Parque Nacional Querétaro 12 Centro y Eje Neovolcánico El Histórico Coyoacán Parque Nacional Ciudad de México Ciudad de México y 13 Centro y Eje Neovolcánico El Tepeyac Parque Nacional Estado de México Morelos y Ciudad de 14 Centro y Eje Neovolcánico El Tepozteco Parque Nacional México 15 Centro y Eje Neovolcánico El Veladero Parque Nacional Guerrero 16 Centro y Eje Neovolcánico Fuentes Brotantes de Tlalpan Parque Nacional Ciudad de México 17 Centro y Eje Neovolcánico General Juan Álvarez Parque Nacional Guerrero 18 Centro y Eje Neovolcánico -

Rare and Endangered
AMERICAN CONIFER SOCIETY coniferQUARTERLY PAGE 13 Rare and Endangered SAVE THE DATES: The American Conifer Society National Meeting June 14 - 17, 2018 Summer 2017 Volume 34, Number 3 CONIFERQUARTERLY (ISSN 8755-0490) is published quarterly by the American Conifer Society. The Society is a non-profit organization incorporated under the laws of the CONIFER Commonwealth of Pennsylvania and is tax exempt under section 501(c)3 of the Internal Revenue Service Code. QUARTERLY You are invited to join our Society. Please address Editor membership and other inquiries to the American Conifer Society National Office, PO Box 1583, Minneapolis, MN Ronald J. Elardo 55311, [email protected]. Membership: US & Canada $40, International $58 (indiv.), $30 (institutional), $75 Technical Editors (sustaining), $100 (corporate business) and $150 (patron). Steven Courtney If you are moving, please notify the National Office 4 weeks David Olszyk in advance. All editorial and advertising matters should be sent to: Advisory Committee Ron Elardo, 5749 Hunter Ct., Adrian, MI 49221-2471, Tom Neff, Committee Chair (517) 902-7230 or email [email protected] Sara Malone Martin Stone Copyright © 2017, American Conifer Society. All rights reserved. No material contained herein may be reproduced Ronald J. Elardo in any form without prior written permission of the publisher. Evelyn Cox, past Editor Opinions expressed by authors and advertisers are not necessarily those of the Society. Cover Photo Keteleria davidiana Taiwan and SE Note: Hardiness Zone references in CONIFERQUARTERLY are USDA classifications unless otherwise specified. Asia. Photo by Tom Cox. Climate Zone Cwa TABLE OF CONTENTS Florida’s BIG Bald Cypress 4 FROM ASHES to REBIRTH By Ronald J. -

This Report Was Prepared As Part the North American Contribution for the State of the World's Forest Genetic Resources
This report was prepared as part the North American contribution for The State of the World’s Forest Genetic Resources (Commission on Genetic Resources for Food and Agriculture, Food and Agriculture Organization of the United Nations, Rome, 2014 available at: http://www.fao.org/forestry/fgr/64582/en/ and on the CONFORGEN website). This North American report was prepared by: Tannis Beardmore, Natural Resources Canada, Canadian Forest Service – Atlantic Region, Hugh John Fleming Forestry Centre, 1350 Regent St. S. PO 4000, Fredericton, New Brunswick, E3G 5P7. E-mail: [email protected] José Jesús Vargas Hernández, Graduate School of Forest Sciences, Colegio de Postgraduados, Km. 36.5 Carr. México-Texcoco, Montecillo, Edo. de México 56230, Mexico. Randy Johnson, National Program Leader Genetics and Global Change Research 1601 N Kent St., RPC-4, Arlington, Virginia, United States¸ 22209. Javier López-Upton, Graduate School of Forest Sciences, Colegio de Postgraduados, Km. 36.5 Carr. México-Texcoco, Montecillo, Edo. de México 56230, Mexico. Martin Williams, Natural Resources Canada, Canadian Forest Service – Atlantic Region, Hugh John Fleming Forestry Centre, 1350 Regent St. S. PO 4000, Fredericton, New Brunswick, E3G 5P7. E-mail: [email protected] 1 | P a g e North America: Regional Synthesis on the State of the World’s Forest Genetic Resources PART 1 - Regional factsheet: 1.1 Importance of forests to the region’s economy, food security, and climate change adaptation 1.1.1 Regional context North America is the third largest continent, covering 24,346,000 km2 (Food and Agriculture Organisation (FAO), 2011) and consisting of three countries: Canada, Mexico, and the United States of America (USA). -
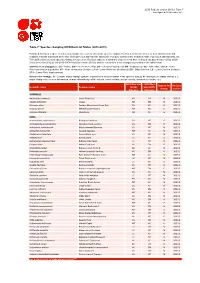
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

Plant Hunting in the USA!
Week Two Plant hunting in the USA! In week two we collected The spruce (figure 4) was Next on the list the beautiful by Luke Wallace Juglans hindsii (northern technically the only species Pinus balfouriana ssb. Californian walnut) and not to be collected from the balfouriana, or foxtail pine, During the three and half week seed-collecting trip to the USA last year, led by Forestry Picea chihuahuana wild. In fact, it was collected which proved to be the Commission Bedgebury and funded by the Friends, a total of 88 species were collected for (chihuahuana spruce). This from a park in the middle second new pine of the trip. several different conservation organisations. Here, we look at some of the more notable walnut (figure 3) is now of a town! However, we In the process of collecting its species that were collected during that trip and what has become of their seeds. only found in the dwindling knew these trees had been seed (figure 5), we stumbled patches of riparian forests planted there 25 years upon a stunning, turquoise Week One that are left in the west earlier from seed collected glacial lake, unlike any lake The most valuable species collected during the coast states. As Westonbirt from a natural population. we had seen before. first week was Pinus albicaulis, the ‘Endangered’* Arboretum holds the As this species is at real risk whitebark pine, mainly threatened in its native range national walnut collection, of extinction in its native by a pathogen known as white pine blister rust. It our Westonbirt companion, Mexico, we were duty-bound was of particular interest to us (as a new pine for the Andy Bryce, was especially to make a collection. -

An Updated List of Species Used in Tree-Ring Research
TREE-RING BULLETIN, Vol. 53, 1993 AN UPDATED LIST OF SPECIES USED IN TREE-RING RESEARCH HENRI D. GRISSINO-MAYER Laboratory of Tree-Ring Research University of Arizona Tucson, AZ 85721, U.S.A. ABSTRACT During the past 100 years, researchers have investigated the potential of hundreds of tree and shrub species for use in applications of tree-ring research. Although several lists of species known to crossdate have been published, investigated species that do not crossdate are rarely included despite the usefulness of this infonnation for future research. This paper provides a list of the Latin and common names of 573 species that have been investigated in tree-ring research, infor mation on species known to crossdate, and information on species with measurement and/or chronology data in the International Tree-Ring Data Bank. In addition, a measure of the suitability of a species for future tree-ring applications, the Crossdating Index (CDI), is developed and pro posed for standard usage. 1n den letzten hundert J ahren haben Forscher das Potential von hunderten von Baum- und Buscharten fi.ir die Anwendung in der Jahresring-Forschung untersucht. Zahlreiche Listen mit Arten, von denen man wei~, da~ sie zeitlich korrespondieren, sind bereits veroffentlicht worden, dagegen sind untersuchte Arten, die nicht zeitlich korresponclieren, selten in Publikationen beriick sichtigt worden, obwohl diese Informationen fi.ir die kiinftige Forschung nutzvoll sein konnten. Dieser Artikel legt eine Liste der lateinischen und der gemeinen Narnen von 573 Arten vor, die im Rahmen der Jahresring-Forschung untersucht worden sind, Inforrnationen Uber Arten, die bekan nterweise zeitlich korrespondieren sowie Informationen iiber Arten mit Ma~- und/oder Chronologiedaten in der intemationalen Jahresring-Datenbank (International Tree-Ring Data Bank). -

Gabriel Eduardo Cervantes Angel Ingeniero Forestal
UNIVERSIDAD AUTÓNOMA AGRARIA ANTONIO NARRO DIVISIÓN DE AGRONOMÍA DEPARTAMENTO FORESTAL Crecimiento de Pináceas Asociadas a Poblaciones Naturales de Picea mexicana Martínez en México Por: GABRIEL EDUARDO CERVANTES ANGEL TESIS Presentada como requisito parcial para obtener el título de: INGENIERO FORESTAL Saltillo, Coahuila, México. Diciembre, 2014 Este proyecto de tesis ha sido apoyado por el Proyecto de Investigación de la Universidad Autónoma Agraria Antonio Narro con No. 38-111-3613-2122, a cargo del profesor investigador Dr. Celestino Flores López. DEDICATORIA A Dios por ser un amigo y compañero, el motivo y guía en mi vida, todo fue posible gracias a su infinito amor, siempre te alabaré. A mis padres: Sra. Irma Angel Clemente Sr. Marcos Cervantes Molina. Quienes me dieron la vida, y procuraron darme alegría desde mi niñez. A ellos les agradezco la persona que hicieron de mí. A mi madre, por su incondicional apoyo en los momentos difíciles y por sus consejos oportunos. A mi padre, por sus regaños, consejos y la paciencia para enseñarme. Son mi orgullo, y siempre estaré agradecido con Dios por ser ustedes mis padres. Son la fortaleza y alegría de mi hogar. A mis hermanos y hermana: Sr. Eddy Ulises Cervantes Angel Sr. Marco Antonio Cervantes Angel Lic. Maricela Cervantes Angel. Quienes siempre han sido mis mejores amigos, en ustedes siempre encontré apoyo incondicional en los momentos difíciles de mi carrera y por su confianza que depositaron en mí. Son parte fundamental de mi vida y son parte de mi logro. A mi novia: Ing. Yocellyn Vázquez Ibarra. Por ser parte de mí vida, ser una persona comprensiva y apoyarme desde el inicio de nuestra hermosa relación, siempre tendrás un espacio muy especial en mi vida. -

The Seed Cone Eathiestrobus Gen. Nov.: Fossil Evidence for a Jurassic
American Journal of Botany 99(4): 708–720. 2012. T HE SEED CONE E ATHIESTROBUS GEN. NOV.: 1 F OSSIL EVIDENCE FOR A JURASSIC ORIGIN OF PINACEAE G AR W . R OTHWELL 2,3,6 , G ENE M APES 2 , R UTH A . S TOCKEY 3,4 AND J ASON H ILTON 5 2 Department of Environmental and Plant Biology, Ohio University, Athens, Ohio 45701 USA; 3 Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon 97331 USA; 4 Department of Biological Sciences, University of Alberta, Edmonton, AB, T6G 2E9, Canada; and 5 School of Geography, Earth and Environmental Sciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK • Premise of the study: Pinaceae and nonpinoid species are sister groups within the conifer clade as inferred from molecular systematic comparisons of living species and therefore should have comparable geological ages. However, the fossil record for the nonpinoid lineage of extant conifer families is Triassic, nearly 100 million years older than the oldest widely accepted Lower Cretaceous record for Pinaceae. An anatomically preserved fossil conifer seed cone described here extends the strati- graphic range of Pinaceae nearly 30 million years, thus reducing the apparent discrepancy between evidence from the fossil record and inferences from systematic studies of living species. • Methods: Material was prepared as serial thin sections by the cellulose acetate peel technique, mounted on microscope slides, and viewed and photographed using transmitted light. • Key results: A large cylindrical cone consisting of bract-scale complexes that diverge from the cone axis in a helical phyllotaxis has bracts and scales that separate from each other in the midregion and are of equal length and of nearly equal width. -

Pdf 419 (Accessed 23 November 2016)
A peer-reviewed version of this preprint was published in PeerJ on 12 June 2017. View the peer-reviewed version (peerj.com/articles/3452), which is the preferred citable publication unless you specifically need to cite this preprint. Dominguez-Guerrero IK, del Rocío Mariscal-Lucero S, Hernández-Díaz JC, Heinze B, Prieto-Ruiz JÁ, Wehenkel C. 2017. Discrimination of Picea chihuahuana Martinez populations on the basis of climatic, edaphic, dendrometric, genetic and population traits. PeerJ 5:e3452 https://doi.org/10.7717/peerj.3452 Discrimination of Picea chihuahuana Martinez populations on the basis of numerous dendrometric, climatic and edaphic traits and genetic diversity Iliana Karina Dominguez-Guerrero 1 , Samantha Mariscal-Lucero 2 , José Ciro Hernández-Díaz 2 , Berthold Heinze 3 , José Ángel Prieto-Ruiz 4 , Christian Wehenkel Corresp. 2 1 Universidad Juárez del Estado de Durango, Durango, Mexico 2 Forestry and Wood Industry Institute, Universidad Juárez del Estado de Durango, Durango, Mexico 3 Federal Research Centre for Forests, Natural Hazards and Landscape (BFW), Vienna, Austria 4 Faculty of Forestry Sciences, Universidad Juárez del Estado de Durango, Durango, Mexico Corresponding Author: Christian Wehenkel Email address: [email protected] Background. Picea chihuahuana, which is endemic to Mexico, is currently listed as “Endangered” on the Red List. Chihuahua spruce is only found in the Sierra Madre Occidental (SMO), Mexico. About 42,600 individuals are distributed in forty populations. The populations are fragmented and can be classified into three distinct clusters in the SMO of the two States (south, center and north), each group separated by a distance of about 300 km. The total area covered P.