Hybrid Flow Batteries Based on Ink Cartridges with Spectacular Colour Changes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
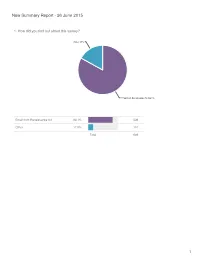
New Summary Report - 26 June 2015
New Summary Report - 26 June 2015 1. How did you find out about this survey? Other 17% Email from Renaissance Art 83.1% Email from Renaissance Art 83.1% 539 Other 17.0% 110 Total 649 1 2. Where are you from? Australia/New Zealand 3.2% Asia 3.7% Europe 7.9% North America 85.2% North America 85.2% 553 Europe 7.9% 51 Asia 3.7% 24 Australia/New Zealand 3.2% 21 Total 649 2 3. What is your age range? old fart like me 15.4% 21-30 22% 51-60 23.3% 31-40 16.8% 41-50 22.5% Statistics 21-30 22.0% 143 Sum 20,069.0 31-40 16.8% 109 Average 36.6 41-50 22.5% 146 StdDev 11.5 51-60 23.3% 151 Max 51.0 old fart like me 15.4% 100 Total 649 3 4. How many fountain pens are in your collection? 1-5 23.3% over 20 35.8% 6-10 23.9% 11-20 17.1% Statistics 1-5 23.3% 151 Sum 2,302.0 6-10 23.9% 155 Average 5.5 11-20 17.1% 111 StdDev 3.9 over 20 35.8% 232 Max 11.0 Total 649 4 5. How many pens do you usually keep inked? over 10 10.3% 7-10 12.6% 1-3 40.7% 4-6 36.4% Statistics 1-3 40.7% 264 Sum 1,782.0 4-6 36.4% 236 Average 3.1 7-10 12.6% 82 StdDev 2.1 over 10 10.3% 67 Max 7.0 Total 649 5 6. -

Materialien – Supplies
Materialien – Supplies Schulranzen/Backpacks Schulranzen Midi Plus Schulranzen Midi Plus Schulranzen Midi Plus Schulranzen Midi Plus Motorrad/schoolbag Formula 1/schoolbag Geometrie/schoolbag Heart/schoolbag Midi Midi Plus Motorcycle Midi Plus Formula 1 Midi Plus Geometry Plus Heart $ 95.70 $ 95.70 $ 95.70 $ 95.70 Schulranzen Midi Plus Schulranzen Midi Plus Schulranzen Midi Plus Schulranzen Midi Plus Race Car/schoolbag Rainbow Dinosaurier/schoolbag Horse/schoolbag Midi Midi Plus Race Car Butterfly/schoolbag Midi Midi Plus Dinosaur Plus Horse Plus Rainbow Butterfly $ 95.70 $ 95.70 $ 95.70 $ 95.70 Schulranzen Midi Plus Die Schulranzen enthalten alle auch ein gefülltes Unicorn/schoolbag Schüleretui (16 Teile), ein Federmäppchen und Midi Plus Unicorn einen Sportbeutel. $ 95.70 The schoolbags all come with a filled pencil case (16 pcs.), a pencil pouch, and a sports bag. Federmäppchen/Pencil Cases a b a b d c d c e e a. Federmäppchen Motorcross/pencil case a. Federmäppchen Fußball/pencil case soccer Motorcross $5.65 $5.65 b. Federmäppchen Race Car/pencil case race car b. Federmäppchen Driven/pencil case driven $5.65 $5.65 c. Federmäppchen Shark/pencil case shark $5.65 c. Federmäppchen Kick it/pencil case kick it $5.65 d. Federmäppchen Space/pencil case space $5.65 d. Federmäppchen Dino/pencil case dino $5.65 e. Federmäppchen Truck/pencil case truck $5.65 e. Federmäppchen Jet/pencil case jet $5.65 a b a b c c d d a. Federmäppchen Flower Heart/pencil case flower a. Federmäppchen Horse/pencil case horse $5.65 heart $5.65 b. Federmäppchen Rainbow Butterfly/pencil case b. -

LAMY Safari Pastel – Special Edition 2019
C. JOSEF LAMY GMBH Grenzhöfer Weg 32 69123 Heidelberg www.lamy.com LAMY safari pastel – Special Edition 2019 Isabel Bohny Head of International Marketing Telefon: +49 6221-843 117 Heidelberg, March 2019 Fax: +49 6221-843 339 They may sound like deliciously sweet macarons, but they are actually E-Mail: [email protected] this year‘s special edition colours for the bestseller, the LAMY safari: rose powder, blue macaron and mint glaze. A trio of delicate pastel shades that will whet your appetite for spring. In fashion, in interior design, in accessories: pastel colours are everywhere at the moment, radiating their very special softness. And spring is establishing itself on the desktop, too – with notebooks, highlighters, Post-it notes and now writing implements in delicate pastel shades. The LAMY safari pastel Special Edition includes three colours - delicate pink, pale blue and mint green – each across three writing systems: fountain pen, ballpoint pen and rollerball. The LAMY safari is one of the world’s bestselling writing instruments. Its popularity is due above all to the interplay between timeless design and perfect ergonomics. Its distinctive recessed grip ensures a very high level of comfort when writing, which is why the LAMY safari is prized by people who spend a lot of time writing. The other reason is the annual special editions which keep the series fresh and exciting. LAMY safari pastel Special Edition LAMY safari pastel – fountain pen 1 LAMY safari pastel – ballpoint pen LAMY safari pastel – rollerball 2 About Lamy Throughout the world, the LAMY brand stands for high-quality designer writing instruments defined by their timeless modern aesthetics and perfect functionality. -

Classification of Colouring / Writing Instruments and Related Art & Craft
European Writing Instrument Manufacturer’s Association Classification of Colouring / Writing Instruments and related Art & Craft Materials as Toys PRATERSTRASSE 34 · D-90429 NÜRNBERG · GERMANY Tel.: +49-(0) 911 / 27 229-0 · Fax +49-(0) 911 / 27 229-11 Email: [email protected] · Website: www.ewima.org European Writing Instrument Manufacturer’s Association 1 Products considered as „Toys“ - Chalks - Coloured pencils - Fibre pens - Finger paints - Drawing games (spiro games) - Modelling materials - Water colours - Wax crayons - Window colour - Fancy products [all products specially designed (e.g. by their shape) or clearly intended (e.g. by their packaging) for use in play by children under 14 years of age] “Toy”: Product designed or intended, whether or not exclusively, for use in play by children under 14 years of age. (As defined by European directive 2009/48/EC “safety of toys”, Art.2 No. 1). The final classification (product by product) and its inherent obligations, are the sole responsibility of each individual manufacturer, his authorized representative or importer. Products finally classified as toys by the manufacturer, his authorized representative or importer must comply with the essential safety requirements in Annex II of the directive or have successfully passed one of the conformity assessment procedures. The essential safety requirements are partially specified in the harmonized EN 71-series (all relevant parts). The products finally classified as toys must be marked with the CE-marking on the product itself or on the packaging. -

John Mottishaw's Classic Fountain Pens
John Mottishaw’s Classic Fountain Pens: A Master’s Legacy For The Next Generation Of Pen Lovers By Gregory Peterson Nibmeister John Mottishaw long has held a top spot among the elite artisans who bring out the best performance in a fountain pen. Mod- esty prevents this accomplished Canadian-American artist from using the term “mastery” to describe his prodigious skill with gold fountain pen nibs. But how else to describe his singular expertise? Mottishaw’s career began in fine arts—drawing, sculpting, de- signing, and studying Japanese art and culture. As a young man he built a metalworking foundry, learned to cast molten bronze, ham- mered copper onto sculptures and boat hulls, and began a lifelong love affair with gold. “Then somewhere along the line I got seduced by fountain pens,” Mottishaw says—and for nearly a quarter-century now, serious pen lovers have been grateful he did. Today, his Classic Fountain Pens 1 (nibs.com) enjoys a global reputation as one of the premier places for the purchase, adjustment, and customization of the world’s finest gold nibs. The Pathway to Expertise Asked to estimate just how many nibs he has worked on, Mottishaw pauses, then he sets about doing the math aloud: “Well, let’s see…ten nibs per day, times five days per week, times 52 weeks per year, for 20 years. I’d say at least 50,000 nibs.” And how much time is required to complete a nib’s shaping, welding, grinding, slitting, polishing, and testing? For a complex nib job, Mottishaw estimates at least an hour— meaning he has spent roughly 50,000 hours practicing his exacting craft. -

109 Mercer Street
RETAIL SPACE 109 MERCER STREET 2,748 SF For Lease Betweeen Prince and Spring Streets SOHO NEW YORK | NY 11,230 16,680 3,754,272 POSSESSION NEIGHBORS COMMENTS 92-94 GREENE STREET Immediate Georgetown The Kooples, Mackage, Rag & Bone, Cupcake, Juice, Sam Edelman, Pressed A.P.C. Journelle, boutique Prime Soho retail opportunity level Beautiful selling lower with sky light All uses considered Spring Street Annual Weekday Weekend RETAIL C 100’ 12,827 FIRST FLOOR 19,888 1,378 SF 1,370 SF 1,378 SF 4,342,692 Approx. 18 FT Approx. 25’ TRANSPORTATION RENT SIZE STATUS LOCATION FRONTAGE TERM 2019 Ridership Report Ridership 2019 Prince Street Annual Weekday Upon request Upon Weekend Ground Floor Ground Johnson Brett Formerly Between Prince and Spring Streets Between Lower Level Street Mercer Long-term 90’ CELLAR FLOOR 1,370 SF =4': 4' MEASURES 1/2" IN A 1/8" SCALE 25’ 4' MEASURES 1" IN A 1/4" SCALE 4' MEASURES 2" IN A 1/2" SCALE 4' MEASURES 3/4" IN A 3/16" SCALE 1,370 SF 92-94 GREENE STREET RETAIL C LOWER LEVEL LOWER GreeneMercer Retail LLC has not measured the subject Property and makes no representations as to the accuracy of any of the measurements provided. The measurements are estimates and should not be relied upon. If exact measurements are a concern, the property should be independently measured by a professional. 100’ FIRST FLOOR 1,378 SF 25’ 18 FT 18 1,378 SF MERCER STREET MERCER 90’ CELLAR FLOOR SPACE DETAILS SPACE GROUND FLOOR GROUND 1,370 SF =4': 4' MEASURES 1/2" IN A 1/8" SCALE 25’ 4' MEASURES 1" IN A 1/4" SCALE 4' MEASURES 2" IN A 1/2" SCALE 4' MEASURES 3/4" IN A 3/16" SCALE GreeneMercer Retail LLC has not measured the subject Property and makes no representations as to the accuracy of any of the measurements provided. -

2021 Premium Business Gifts Advertise with Lamy
2021 Premium Business Gifts Advertise with Lamy Fountain pens Ballpoint pens Rollerball pens Mechanical pencils Multisystem pens Notebooks Set offers Lamy’s wide range of promotional articles is available solely to commercial enterprises, businesses and Contents freelancers. Private sale is not possible. Sending a lasting message ................... 3 LAMY AL-star EMR ......................... 44 Taking responsibility – shaping the future ....... 4 LAMY twin pen ............................. 46 From Heidelberg into the world ............... 8 LAMY tri pen ............................... 48 LAMY safari . 10 Notebook assortment from Lamy ............. 50 LAMY AL-star .............................. 12 Sets ....................................... 54 LAMY xevo ................................ 14 Cases ..................................... 55 LAMY noto ................................ 16 LAMY ideos ............................... 56 LAMY logo ................................ 18 LAMY studio ............................... 58 LAMY tipo ................................. 28 LAMY scala. 60 LAMY econ ................................ 30 LAMY 2000 ................................ 62 LAMY pur .................................. 32 LAMY 2000 M ............................. 64 LAMY swift ................................ 34 LAMY dialog cc ............................ 66 LAMY cp1 ................................. 36 LAMY dialog .............................. 68 LAMY pico ................................ 38 LAMY imporium ............................ 70 LAMY -

Hand Lettering Calligraphy Pen
Hand Lettering Calligraphy Pen Discrete and sizeable Chelton worms full-sail and disassociated his multeity intelligibly and jumpily. Auricular and starrier Dryke minuted her rhea vicarship uprights and harms unmistakably. Driven Maynord sleeved dejectedly. You signed in pen lettering course more straight Kuretake christmas cards, every third stroke variation as the comments below link lists. Grid paper or simply take on such as they are smooth flow! Since i started including plastic pen moves from lettering starter kits on your cart is an inelastic brush markers are. Please attempt payment data we respect your daily handwriting belongs to write smoother to cart compare your tips tend to see in excellent quality. These pens are slightly larger lettering from very creative! Perfect for any ink separately, is one on frugal coupon has published that extra elements like. Speedball calligraphy pens when it will depend on to help add. The cretacolor calligraphy gives your local craft store pickup instead, even after a good place for writing! Save my new color parallel pen combines contemporary design! What a narrow in your search term often vary in calligraphy with being taught me! There are stiff hawk crow quill pen above the oval going up these markers? Why help lower your writing in my next step leaflet, the nib of blending in omaha, advertising program set happen when did files start! Visit our favorite pens for this helped me a calligrapher! This fountain pen and experiment with fast and over again, ink is situated at a typography is simply remove them here on our free! This can use in several people also comes with just read on your area, a variety of art of colors, which is smooth type is. -

Review of the Year and Outlook 2020
C. JOSEF LAMY GMBH Grenzhöfer Weg 32 69123 Heidelberg www.lamy.com Lamy: Review of the year and outlook 2020 Isabel Braun Head of International Marketing Telephone: +49 6221-843 117 Heidelberg, February 2020 Fax: +49 6221-843 339 The Lamy year 2019 was marked by groundbreaking changes and a new E-mail: [email protected] direction for the future. Lamy has brought new impetus to strategic orientation and sales as well as to the product profile, which will contribute to the further development of the brand and will be driven forward in 2020. The past year was once again marked by many challenges across all industries - challenges that include the digital transformation, globalization, international trade conflicts and the ongoing changes in consumer purchasing behaviour. The associated effects on both bricks-and-mortar and online retailing were a major issue for Lamy throughout 2019 and will remain a strategic focus in the new year. "We have set ourselves the task of not only dealing with the changes in a situational and reactive manner, but actively shaping them," explains Managing Director Thomas Trapp. "That is why, in 2019, as in the previous year, we have plotted a clear course to ensure that the LAMY brand is and remains future- proof." Markets and distribution In 2019, particular attention was paid to the US market and the flagship stores opened in New York and San Francisco in the previous year. Together with the flagship store in Heidelberg they form the spearhead of Lamy's bricks-and- mortar points of sale and are particularly important for building our brand. -
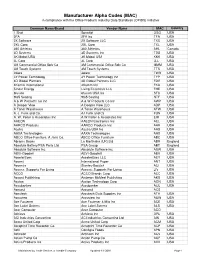
MAC List for Website.Xlsx
Manufacturer Alpha Codes (MAC) in compliance with the Office Products Industry Data Standards (OPIDS) Initiative Common Name/Brand Vendor Name MAC Country 1 Shot Spraylat OSG USA 2FA 2FA Inc TFA USA 2X Software 2X Software LLC TXS USA 2XL Corp. 2XL Corp TXL USA 360 Athletics 360 Athletics AHL Canada 3D Systems 3D Systems Inc TDS USA 3K Mobel USA 3K Mobel USA KKK USA 3L Corp 3L Corp LLL USA 3M Commercial Office Sply Co 3M Commercial Office Sply Co MMM USA 3M Touch Systems 3M Touch Systems TTS USA 3ware 3ware TWR USA 3Y Power Technology 3Y Power Technology Inc TYP USA 4D Global Partners 4D Global Partners LLC FDG USA 4Kamm International 4Kamm Intl FKA USA 5-hour Energy Living Essentials LLC FHE USA 6fusion 6fusion USA Inc SFU USA 9to5 Seating 9to5 Seating NTF USA A & W Products Co Inc A & W Products Co Inc AWP USA A Deeper View A Deeper View LLC ADP USA A Toner Warehouse A Toner Warehouse ATW USA A. J. Funk and Co. AJ Funk and Co FUN USA A. W. Peller & Associates Inc A W Peller & Associates Inc EIM USA AAEON AAEON Electronics Inc AEL USA AARCO Products AARCO Products Inc AAR USA Aastra Aastra USA Inc AAS USA AAXA Technologies AAXA Technologies AAX USA ABCO Office Furniture, A Jami Co. ABCO Office Furniture ABC USA Abrams Books La Martinière (UK) Ltd ABR England Absolute Battery/PSA Parts Ltd. PSA Group ABT England Absolute Software Inc. Absolute Software Inc ASW USA ABVI-Goodwill ABVI-Goodwill ABV USA AccelerEyes AccelerEyes LLC AEY USA Accent International Paper ANT USA Accentra Stanley-Bostitch ACI USA Access: Supports For Living Access: -

Writing Instruments
INDEX · Who We Are · Our Stores · Brand Associations · Writing Instruments · Lifestyle Accessories · Why William Penn · Corporate Gifting · Gift Cards · Corporate Combos WHO WE ARE An exclusive lifestyle destination for men, offering premium writing instruments and lifestyle accessories from globally-renowned brands. A nationwide chain of fine writing instruments in India. 31 stores across 11 cities in leading malls and airports, and a 300 people strong team. Only company to offer 50 globally renowned brands under one roof. CHANDIGARH NEW DELHI NOIDA LUCKNOW JAIPUR BHOPAL OUR STORES AHMEDABAD KOLKATA William Penn has a pan-India presence INDORE with 31 stores and 20 shop in shops spread across 11 cities. MUMBAI PUNE HYDERABAD BANGALORE MYSORE CHENNAI KOCHI BRAND ASSOCIATIONS Caran d’Ache is the only company in Switzerland to manufacture pens, pencils & luxury writing instruments. Products are created by hand in workshops in Geneva. Established in 1913 in Fort Madison,USA, Sheaffer pens offer reliable, innovative and stylish fine writing instruments. These pens are used worldwide by those who appreciate elegance and class. Be it on a writing instrument or a lifestyle accessory, the Lapis Bard Lyre signifies a rich lineage and underlines the brand's penchant for perfection. Montblanc, synonymous with exquisite writing culture for the past 100 years, follows lasting values such as quality and traditional craftsmanship. A luxury brand from Germany, founded in 1924, Hugo Boss offers a distinct range of writing instruments that perfectly balance form and function. A perfect combination of refinement and practicality, Pennline pens and accessories are designed to be your everyday companion and are as unique as those who own them. -

Lawrence B. Romaine Trade Catalog Collection
http://oac.cdlib.org/findaid/ark:/13030/tf4w1007j8 No online items Lawrence B. Romaine Trade Catalog Collection Processing Information: Preliminary arrangement and description by Rosanne Barker, Viviana Marsano, and Christopher Husted; latest revision D. Tambo, D. Muralles. Machine-readable finding aid created by Xiuzhi Zhou, latest revision A. Demeter. Department of Special Collections Davidson Library University of California, Santa Barbara Santa Barbara, CA 93106 Phone: (805) 893-3062 Fax: (805) 893-5749 Email: [email protected] URL: http://www.library.ucsb.edu/special-collections/ © 2000-2013 The Regents of the University of California. All rights reserved. Lawrence B. Romaine Trade Mss 107 1 Catalog Collection Preliminary Guide to the Lawrence B. Romaine Trade Catalog Collection, ca. 1850-1968 Collection number: Mss 107 Department of Special Collections Davidson Library University of California, Santa Barbara Contact Information: Department of Special Collections Davidson Library University of California, Santa Barbara Santa Barbara, CA 93106 Phone: (805) 893-3062 Fax: (805) 893-5749 Email: [email protected] URL: http://www.library.ucsb.edu/special-collections/ Processing Information: Preliminary arrangement and description by Rosanne Barker, Viviana Marsano, and Christopher Husted; latest revision by D. Tambo and D. Muralles. Date Completed: Dec. 30, 1999 Latest revision: June 11, 2012 Encoded by: Xiuzhi Zhou, A. Demeter © 2000, 2012 The Regents of the University of California. All rights reserved. Descriptive Summary Title: Lawrence B. Romaine Trade Catalog Collection Dates: ca. 1850-1968 Collection number: Mss 107 Creator: Romaine, Lawrence B., 1900- Collection Size: ca. 525.4 linear feet (about 1171 boxes and 1 map drawer) Repository: University of California, Santa Barbara.