The Toxicology of the Newer Metals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cobalt Toxicity in Humans. a Review of the Potential Sources and Systemic Health Effects
Cobalt toxicity in humans. A review of the potential sources and systemic health effects. Laura Leyssensa, Bart Vincka,b, Catherine Van Der Straetenc,d, Floris Wuytse,f, Leen Maesa,g. a Faculty of Medicine and Health Sciences, University of Ghent (Belgium) Department of Speech, Language and Hearing Sciences University Hospital Ghent, policlinic 1 floor 2 De Pintelaan 185 9000 Ghent Belgium [email protected] (corresponding author) [email protected] b Faculty of Humanities, University of Pretoria (South Africa) Department of Speech-Language Pathology and Audiology Aula Theatre, University Road Pretoria, 0001 South Africa [email protected] c Faculty of Medicine, Imperial College London Department of Surgery & Cancer Musculoskeletal Sciences and Technology Imperial College London Charing Cross Campus, 7L21 Lab Block London SW7 2AZ UK [email protected] d Faculty of Medicine and Health Sciences, University of Ghent (Belgium) De Pintelaan 185 9000 Ghent Belgium e Antwerp University Research center for Equilibrium and Aerospace (AUREA) Department of Otorhinolaryngology University Hospital Antwerp Campus Groenenborger Groenenborgerlaan 171 2020 Antwerp Belgium [email protected] f Department of Biomedical Physics, University of Antwerp (Belgium) Campus Groenenborger Groenenborgerlaan 171 2020 Antwerp Belgium g Clinical audiology department University Hospital Ghent De Pintelaan 185 9000 Ghent Belgium 1 Abstract Cobalt (Co) and its compounds are widely distributed in nature and are part of numerous anthropogenic activities. Although cobalt has a biologically necessary role as metal constituent of vitamin B12, excessive exposure has been shown to induce various adverse health effects. This review provides an extended overview of the possible Co sources and related intake routes, the detection and quantification methods for Co intake and the interpretation thereof, and the reported health effects. -

A Comparison of Composite Transparent Conducting Oxides Based on the Binary Compounds Cdo and Sno2
October 2001 • NREL/CP-520-31017 A Comparison of Composite Transparent Conducting Oxides Based on the Binary Compounds CdO and SnO2 Preprint X. Li, T. Gessert, C. DeHart, T. Barnes, H. Moutinho, Y. Yan, D. Young, M. Young, J. Perkins, and T. Coutts To be presented at the NCPV Program Review Meeting Lakewood, Colorado 14-17 October 2001 National Renewable Energy Laboratory 1617 Cole Boulevard Golden, Colorado 80401-3393 NREL is a U.S. Department of Energy Laboratory Operated by Midwest Research Institute • Battelle • Bechtel Contract No. DE-AC36-99-GO10337 NOTICE The submitted manuscript has been offered by an employee of the Midwest Research Institute (MRI), a contractor of the US Government under Contract No. DE-AC36-99GO10337. Accordingly, the US Government and MRI retain a nonexclusive royalty-free license to publish or reproduce the published form of this contribution, or allow others to do so, for US Government purposes. This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof. -

United States Patent (19) 11 Patent Number: 4,496,778 Myers Et Al
United States Patent (19) 11 Patent Number: 4,496,778 Myers et al. (45) Date of Patent: Jan. 29, 1985 (54) PROCESS FOR THE HYDROXYLATION OF 56 References Cited OLEFINS USING MOLECULAR OXYGEN, U.S. PATENT DOCUMENTS ANOSMIUM CONTAINING CATALYST, A COPPER CO-CATALYST, AND AN 2,773, 101 12/1956 Smith et al. ......................... 568/860 AROMATIC AMINE BASED PROMOTER 3,317,592 5/1967 Maclean et al. ... 568/860 3,337,635 8/1967 Norton et al. ....... 568/860 75 Inventors: Richard S. Myers, Fairlawn; Robert 4,390,739 6/1983 Michaelson et al. .... ..., 568/860 C. Michaelson, Waldwick; Richard FOREIGN PATENT DOCUMENTS G. Austin, Ridgewood, all of N.J. 32522 8/1974 Japan ................................... 568/860 73) Assignee: Exxon Research & Engineering Co., Primary Examiner-J. E. Evans Florham Park, N.J. Attorney, Agent, or Firm-Robert A. Maggio 21 Appl. No.: 538,190 57 ABSTRACT A process directed to the hydroxylation of olefins by 22 Filed: Oct. 3, 1983 reacting said olefins in the presence of oxygen, water, and a catalyst composition comprising (i) a catalytically 51 Int. Cl. ...................... C07C 29/04; CO7C 31/18; active osmium containing compound, (ii) a Co-catalyst C07C 31/22; CO7C 31/42 I comprising a copper containing compound such as 52 U.S.C. ................................. 568/860; 260/.397.2; CuBr2, and (iii) a Co-catalyst II capable of increasing 560/186; 562/587; 568/811; 568/821; 568/833; the rate and/or selectivity of the hydroxylation reac 568/838; 568/847 tion, such as pyridine is disclosed. 58 Field of Search .............. -

Cellular Uptake and Toxicological Effects of Differently Sized Zinc Oxide Nanoparticles in Intestinal Cells †
toxics Article Cellular Uptake and Toxicological Effects of Differently Sized Zinc Oxide Nanoparticles in Intestinal Cells † Anna Mittag 1,* , Christian Hoera 2, Alexander Kämpfe 2 , Martin Westermann 3, Jochen Kuckelkorn 4, Thomas Schneider 1 and Michael Glei 1 1 Department of Nutritional Toxicology, Institute of Nutritional Sciences, Friedrich Schiller University Jena, Dornburger Straße 24, 07743 Jena, Germany; [email protected] (T.S.); [email protected] (M.G.) 2 German Environment Agency, Swimming Pool Water, Chemical Analytics, Heinrich-Heine-Straße 12, 08645 Bad Elster, Germany; [email protected] (C.H.); [email protected] (A.K.) 3 Electron Microscopy Centre, Friedrich Schiller University Jena, Ziegelmühlenweg 1, 07743 Jena, Germany; [email protected] 4 German Environment Agency, Toxicology of Drinking Water and Swimming Pool Water, Heinrich-Heine-Straße 12, 08645 Bad Elster, Germany; [email protected] * Correspondence: [email protected] † In respectful memory of Dr. Tamara Grummt. Abstract: Due to their beneficial properties, the use of zinc oxide nanoparticles (ZnO NP) is constantly increasing, especially in consumer-related areas, such as food packaging and food additives, which is leading to an increased oral uptake of ZnO NP. Consequently, the aim of our study was to investigate the cellular uptake of two differently sized ZnO NP (<50 nm and <100 nm; 12–1229 µmol/L) using two human intestinal cell lines (Caco-2 and LT97) and to examine the possible resulting toxic effects. ZnO NP (<50 nm and <100 nm) were internalized by both cell lines and led to intracellular changes. Citation: Mittag, A.; Hoera, C.; Kämpfe, A.; Westermann, M.; Both ZnO NP caused time- and dose-dependent cytotoxic effects, especially at concentrations of Kuckelkorn, J.; Schneider, T.; Glei, M. -

Cobalt-Nickel Strip, Plate, Bar, and Tube Safety Data Sheet Revision Date: 12/14/2012
Cobalt-Nickel Strip, Plate, Bar, and Tube Safety Data Sheet Revision date: 12/14/2012 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product name. : Cobalt-Nickel Strip, Plate, Bar, and Tube 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/preparation : Parts Manufacturing No additi onal infor mati on available 1.3. Details of the supplier of the safety data sheet Ametek Specialty Metal Products 21 Toelles Road Wallingford, CT 06492 T 203-265-6731 1.4. Emergency telephone number Emergency number : 800-424-9300 Chemtrec SECTION 2: Hazards identification 2.1. Classification of the substance or mixture GHS-US classification Comb. Dust H232 Resp. Sens. 1 H334 Skin Sens. 1 H317 Carc. 2 H351 STOT RE 1 H372 STOT RE 2 H373 Aquatic Acute 1 H400 Aquatic Chronic 4 H413 2.2. Label elements GHS-US labelling Hazard pictograms (GHS-US) : Signal word (GHS-US) : Danger Hazard statements (GHS-US) : H232 - May form combustible dust concentrations in air H317 - May cause an allergic skin reaction H334 - May cause allergy or asthma symptoms or breathing difficulties if inhaled H351 - Suspected of causing cancer H372 - Causes damage to organs through prolonged or repeated exposure H373 - May cause damage to organs through prolonged or repeated exposure H400 - Very toxic to aquatic life H413 - May cause long lasting harmful effects to aquatic life 12/14/2012 EN (English) 1/9 Cobalt-Nickel Strip, Plate, Bar, and Tube Safety Data Sheet Precautionary statements (GHS-US) : P201 - Obtain special instructions before use P202 - Do not handle until all safety precautions have been read and understood P260 - Do not breathe dust/fume/gas/mist/vapours/spray P264 - Wash .. -

The Two Faces of Titanium Dioxide Nanoparticles Bio-Camouflage in 3D
www.nature.com/scientificreports OPEN The two faces of titanium dioxide nanoparticles bio-camoufage in 3D bone spheroids Received: 23 October 2018 W. Souza1,2,3, S. G. Piperni3,4, P. Laviola1,3,5, A. L. Rossi4, Maria Isabel D. Rossi6, Accepted: 11 June 2019 Bráulio S. Archanjo7, P. E. Leite 1,2,8, M. H. Fernandes9,12, L. A. Rocha3,10, J. M. Granjeiro1,2,3,11 Published: xx xx xxxx & A. R. Ribeiro 2,3,5 Titanium (Ti) and its alloys are widely used in dental implants and hip-prostheses due to their excellent biocompatibility. Growing evidence support that surface degradation due to corrosion and wear processes, contribute to implant failure, since the release of metallic ions and wear particles generate local tissue reactions (peri-implant infammatory reactions). The generated ions and wear debris (particles at the micron and nanoscale) stay, in a frst moment, at the interface implant-bone. However, depending on their size, they can enter blood circulation possibly contributing to systemic reactions and toxicities. Most of the nanotoxicological studies with titanium dioxide nanoparticles (TiO2 NPs) use conventional two-dimensional cell culture monolayers to explore macrophage and monocyte activation, where limited information regarding bone cells is available. Recently three- dimensional models have been gaining prominence since they present a greater anatomical and physiological relevance. Taking this into consideration, in this work we developed a human osteoblast- like spheroid model, which closely mimics bone cell-cell interactions, providing a more realistic scenario for nanotoxicological studies. The treatment of spheroids with diferent concentrations of TiO2 NPs during 72 h did not change their viability signifcantly. -

CLINICAL TOXICOLOGY THROUGH the AGES Programme 11Th November 2016 Aula of the University of Zürich Kol G 201, Rämistrasse 71, 8006 Zürich
Anniversary Symposium 50 Years Tox Info Suisse CLINICAL TOXICOLOGY THROUGH THE AGES Programme 11th November 2016 Aula of the University of Zürich Kol G 201, Rämistrasse 71, 8006 Zürich Anniversary Symposium 50 Years Tox Info Suisse CLINICAL TOXICOLOGY THROUGH THE AGES Part 1: Humans and Animals Chair: Hugo Kupferschmidt 12:15 Opening Hugo Kupferschmidt, Director Tox Info Suisse 12:20 Differing aspects in human and veterinary toxicology Hanspeter Nägeli, Zürich 12:55 Food poisoning today - current research to target old problems Martin J. Loessner, Zürich 13:30 Venomous animals in Switzerland Jürg Meier, Basel 14:05 Coffee break Part 2: Hips, Pain Killers and Mushrooms Chair: Michael Arand 14:35 Welcome address Michael O. Hengartner, President UZH 14:45 Fatal shoot from the hip: News of heavy metal poisoning Sally Bradberry, Birmingham UK 15:20 Old and new aspects in paracetamol poisoning D. Nicholas Bateman, Edinburgh 15:55 Amanita phalloïdes poisoning Thomas Zilker, München 16:30 A silent threat - chronic intoxications Michael Arand, Zürich 17:05 Coffee break Part 3: From Critical Care to the Opera Chair: Martin Wilks 17:35 Management of severe poisoning-induced cardiovascular compromise Bruno Mégarbane, Paris 18:10 Chemical terrorism: New and old chemical weapons and their counter- measures Horst Thiermann, München 18:45 Novel psychoactive substances: How much a threat in public health? Alessandro Ceschi, Lugano 19:20 Poisons in the opera Alexander Campbell, Birmingham UK 19:50 Conclusion 20:00 Apéro riche 21:30 Closure 50 Years Tox Info Suisse | Anniversary Symposium | 11th November 2016 2/15 Anniversary Symposium 50 Years Tox Info Suisse CLINICAL TOXICOLOGY THROUGH THE AGES Speakers Hanspeter Nägeli Differing aspects in human and veterinary toxicology Institute of Veterinary Pharma- Veterinary toxicology is a difficult, yet fascinating subject as it cology and Toxicology, deals with multiple species and a wide variety of poisons of very University of Zurich diverse origins. -

United States Patent Office Attented Oct
3,536,479 United States Patent Office attented Oct. 27, 1970 2 a hydrogen pressure of at least about 15 pounds per 3,536,479 square inch gauge (p.S.i.g.). METHOD FOR THE PRODUCTION OF HIGH PURTY (OSMUM The concentrated osmium-containing solution can ad Alexander Ellis, Clarkson, Ontario, and Alan Manson, vantageously be prepared from a dilute osmium-contain Oakville, Ontario, Canada, assignors to The Interna 5 ing solution as described hereinafter. However, most gen tional Nicke Company, Inc., New York, N.Y., a cor erally the concentrated solution will be prepared by poration of Delaware scrubbing gases containing osmium tetroxide with a Solu No Drawing. Filled Dec. 13, 1967, Ser. No. 690,083 tion containing, by weight, about 5% to 40% sodium hy Claims priority, application Canada, Feb. 21, 1967, droxide to produce a sodium perosmate solution which 983,421 10 contains about 5 grams per liter (gp.l.) to about 100 nt. C. C22b 7/00, 11/04 g.p.l. of osmium, e.g., about 60 g.p.l. of osmium. Gen U.S. C. 75-108 29 Claims erally at this stage only minor amounts of ruthenium, i.e., less than about 0.5 g.p.l. of ruthenium, accompany the osmium and can be precipitated with only minor ABSTRACT OF THE DISCLOSURE 5 amounts of osmium occluded therein from the concen Metallic osmium is recovered from a slurry of an trated osmium-containing solution by adding a Water osmium-containing material to which sufficient hydro soluble, mild organic reducing agent such as methyl al chloric acid has been added to assure a final pH value cohol and ethyl alcohol. -
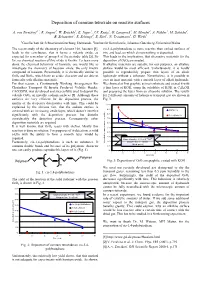
Deposition of Osmium Tetroxide on Reactive Surfaces
Deposition of osmium tetroxide on reactive surfaces A. von Zweidorf1,2, R. Angert1, W. Brüchle1, E. Jäger1, J.V. Kratz2, G. Langrock2, M. Mendel2, A. Nähler2, M. Schädel1, B. Schausten1, E. Schimpf1, E. Stiel1, N. Trautmann2, G. Wirth1 1Gesellschaft für Schwerionenforschung, Darmstadt, 2Institut für Kernchemie, Johannes Gutenberg-Universität Mainz The recent study of the chemistry of element 108, hassium [1], cis-1,4-polybutadiene is more reactive than etched surfaces of leads to the conclusion, that it forms a volatile oxide, as zinc and lead, on which almost nothing is deposited. expected for a member of group 8 of the periodic table [2]. So This leads to the implication, that alternative materials for the far, no chemical reaction of this oxide is known. To learn more deposition of OsO4 are needed. about the chemical behaviour of hassium, one would like to If alkaline materials are suitable for our purposes, an alkaline investigate the chemistry of hassium oxide, the only known surface would be most efficient. Unfortunately, it is hardly compound of hassium. Presumably, it is chemically similar to possible to reproducibly prepare thin layers of an alkali OsO4 and RuO4, which have an acidic character and are able to hydroxide without a substrate. Nevertheless, is it possible to form salts with alkaline materials. coat an inert material with a smooth layer of alkali hydroxide. For that reason, a Continuously Working Arrangement For We choosed at first graphite as inert substrate and coated it with Clusterless Transport Of In-situ Produced Volatile Oxides, a thin layer of KOH, using the solubility of KOH in C2H5OH CALLISTO, was developed and successfully used to deposit the and preparing the layer from an ethanolic solution. -

Particularly Hazardous Substances
Particularly Hazardous Substances In its Laboratory Standard, OSHA requires the establishment of additional protections for persons working with "Particularly Hazardous Substances" (PHS). OSHA defines these materials as "select" carcinogens, reproductive toxins and acutely toxic materials. Should you wish to add: explosive, violently reactive, pyrophoric and water-reactve materials to this category, the information is included. Carbon nanotubes have also been added due to their suspected carcinogenic properties. This table is designed to assist the laboratory in the identification of PHS, although it is not definitively conclusive or entirely comprehensive. *Notes on the proper use of this table appear on page 12. 1 6 5 2 3 4 Substance CAS National Toxicity National Program Carcinogen Toxin Acute Regulated OSHA Carcinogen Group IARC Carcinogen Toxin Reproductive Violently Reactive/ Explosive/Peroxide Forming/Pyrophoric A-a-C(2-Amino-9H-pyrido[2,3,b]indole) 2648-68-5 2B Acetal 105-57-7 yes Acetaldehyde 75-07-0 NTP AT 2B Acrolein (2-Propenal) 107-02-8 AT Acetamide 126850-14-4 2B 2-Acetylaminofluorene 53-96-3 NTP ORC Acrylamide 79-06-6 NTP 2B Acrylyl Chloride 814-68-6 AT Acrylonitrile 107-13-1 NTP ORC 2B Adriamycin 23214-92-8 NTP 2A Aflatoxins 1402-68-2 NTP 1 Allylamine 107-11-9 AT Alkylaluminums varies AT Allyl Chloride 107-05-1 AT ortho-Aminoazotoluene 97-56-3 NTP 2B para-aminoazobenzene 60-09-3 2B 4-Aminobiphenyl 92-67-1 NTP ORC 1 1-Amino-2-Methylanthraquinone 82-28-0 NTP (2-Amino-6-methyldipyrido[1,2-a:3’,2’-d]imidazole) 67730-11-4 2B -

Biological Monitoring of Chemical Exposure in the Workplace Guidelines
WHO/HPR/OCH 96.2 Distr.: General Biological Monitoring of Chemical Exposure in the Workplace Guidelines Volume 2 World Health Organization Geneva 1996 Contribution to the International Programme on Chemical Safety (IPCS) Layout of the cover page Tuula Solasaari-Pekki Technical editing Suvi Lehtinen This publication has been published with the support of the Finnish Institute of Occupational Health. ISBN 951-802-167-8 Geneva 1996 This document is not a formal publication Ce document n'est pas une publication of of the World Health Organization (WHO), ficielle de !'Organisation mondiale de la and all rights are reserved by the Organiza Sante (OMS) et tous Jes droits y afferents tion. The document may, however, be sont reserves par !'Organisation. S'il peut freely reviewed, abstracted, reproduced and etre commente, resume, reproduit OU translated, in part or in whole, but not for traduit, partiellement ou en totalite, ii ne sale nor for use in conjunction with .com saurait cependant l'etre pour la vente ou a mercial purposes. des fins commerciales. The views expressed in documents by Les opinions exprimees clans Jes documents named authors are solely the responsibility par des auteurs cites nommement n'enga of those authors. gent que lesdits auteurs. Preface This is the second in a series of volumes on 'Guidelines on Biological Monitoring of Chemical Exposure in the Workplace', produced under the joint direction of WHO's Of fice of Occupational Health (OCH) and Programme for the Promotion of Chemical Safety (PCS). The objectives of this project was to provide occupational health professionals in Mem ber States with reference principles and methods for the determination of biomarkers of exposure, with emphasis on promoting appropriate use of biological monitoring and as sisting in quality assurance. -

The Preparation of Polysiloxanes from Halosilanes
European Patent Office © Publication number: 0 205 066 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION © Application number: 86107474.8 © Int. CI.4: C 08 G 77/08 © Date of filing: 02.06.86 © Priority: 14.06.85 US 744708 © Applicant: DOW CORNING CORPORATION P.O. Box 1767 Midland Michigan 48640(US) © Date of publication of application: 17.12.86 Bulletin 86/51 @ Inventor: Marko, Ollie William Route 2 Box 60 © Designated Contracting States: Carrollton Kentucky(US) DE FR GB @ Inventor: Steinmeyer, Robert David 214 Deatherage Drive Carrollton Kentucky(US) © Inventor: Rentsch, Stefan Felix 5800 Siebert Midland Michigan(US) © Representative: Sternagel, Hans-Gunther, Dr. et al, Patentanwalte Dr. Michael Hann Dr. H.-G. Sternagel Sander Aue30 D-5060 Bergisch Gladbach 2(DE) © The preparation of polysiloxanes from halosilanes. ©@ This This invention relates to a process for the preparation of polysiloxanes by reacting halosilanes in the presence of metal oxides and sulfolane. Preferred metal oxides include anti- mony (III) oxide, antimony (V) oxide, cadmium oxide, calcium oxide, copper (II) oxide, indium oxide, iron (II) oxide, iron (III) oxide, magnesium oxide, manganese (II) oxide, mercury (II) oxide, nickel (II) oxide, thallium (III) oxide, tin (II) oxide, and zinc oxide. Improved yields and rates of reaction can be observed with the process of this invention. Croydon Printing Company Ltd. This invention relates to a process for the preparation of polysiloxanes from halosilanes. 14ore specifically, this invention relates to a process for the preparation of polysiloxanes by reacting halosilanes in the presence of metal oxides and sulfolane. Polysiloxanes are most commonly prepared by the hydrolysis of halosilanes.