Expression and Role of VEGF-A in the Ciliary Body
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Distribution of Immune Cells in the Uveal Tract of the Normal Eye
THE DISTRIBUTION OF IMMUNE CELLS IN THE UVEAL TRACT OF THE NORMAL EYE PAUL G. McMENAMIN Perth, Western Australia SUMMARY function of these cells in the normal iris, ciliary body Inflammatory and immune-mediated diseases of the and choroid. The role of such cell types in ocular eye are not purely the consequence of infiltrating inflammation, which will be discussed by other inflammatory cells but may be initiated or propagated authors in this issue, is not the major focus of this by immune cells which are resident or trafficking review; however, a few issues will be briefly through the normal eye. The uveal tract in particular considered where appropriate. is the major site of many such cells, including resident tissue macro phages, dendritic cells and mast cells. This MACRO PHAGES review considers the distribution and location of these and other cells in the iris, ciliary body and choroid in Mononuclear phagocytes arise from bone marrow the normal eye. The uveal tract contains rich networks precursors and after a brief journey in the blood as of both resident macrophages and MHe class 11+ monocytes immigrate into tissues to become macro dendritic cells. The latter appear strategically located to phages. In their mature form they are widely act as sentinels for capturing and sampling blood-borne distributed throughout the body. Macrophages are and intraocular antigens. Large numbers of mast cells professional phagocytes and play a pivotal role as are present in the choroid of most species but are effector cells in cell-mediated immunity and inflam virtually absent from the anterior uvea in many mation.1 In addition, due to their active secretion of a laboratory animals; however, the human iris does range of important biologically active molecules such contain mast cells. -

Ciliary Zonule Sclera (Suspensory Choroid Ligament)
ACTIVITIES Complete Diagrams PNS 18 and 19 Complete PNS 23 Worksheet 3 #1 only Complete PNS 24 Practice Quiz THE SPECIAL SENSES Introduction Vision RECEPTORS Structures designed to respond to stimuli Variable complexity GENERAL PROPERTIES OF RECEPTORS Transducers Receptor potential Generator potential GENERAL PROPERTIES OF RECEPTORS Stimulus causing receptor potentials Generator potential in afferent neuron Nerve impulse SENSATION AND PERCEPTION Stimulatory input Conscious level = perception Awareness = sensation GENERAL PROPERTIES OF RECEPTORS Information conveyed by receptors . Modality . Location . Intensity . Duration ADAPTATION Reduction in rate of impulse transmission when stimulus is prolonged CLASSIFICATION OF RECEPTORS Stimulus Modality . Chemoreceptors . Thermoreceptors . Nociceptors . Mechanoreceptors . Photoreceptors CLASSIFICATION OF RECEPTORS Origin of stimuli . Exteroceptors . Interoceptors . Proprioceptors SPECIAL SENSES Vision Hearing Olfaction Gustation VISION INTRODUCTION 70% of all sensory receptors are in the eye Nearly half of the cerebral cortex is involved in processing visual information Optic nerve is one of body’s largest nerve tracts VISION INTRODUCTION The eye is a photoreceptor organ Refraction Conversion (transduction) of light into AP’s Information is interpreted in cerebral cortex Eyebrow Eyelid Eyelashes Site where conjunctiva merges with cornea Palpebral fissure Lateral commissure Eyelid Medial commissure (a) Surface anatomy of the right eye Figure 15.1a Orbicularis oculi muscle -

The Proteomes of the Human Eye, a Highly Compartmentalized Organ
Proteomics 17, 1–2, 2017, 1600340 DOI 10.1002/pmic.201600340 (1 of 3) 1600340 The proteomes of the human eye, a highly compartmentalized organ Gilbert S. Omenn Center for Computational Medicine and Bioinformatics, University of Michigan, Ann Arbor, MI, USA Proteomics has now published a series of Dataset Briefs on the EyeOme from the HUPO Received: November 2, 2016 Human Proteome Project with high-quality analyses of the proteomes of these compartments Accepted: November 4, 2016 of the human eye: retina, iris, ciliary body, retinal pigment epithelium/choroid, retrobulbar optic nerve, and sclera, with 3436, 2929, 2867, 2755, 2711, and 1945 proteins, respectively. These proteomics resources represent a useful starting point for a broad range of research aimed at developing preventive and therapeutic interventions for the various causes of blindness. Keywords: Biomedicine / Biology and Disease-driven Human Proteome Project / End Blindness by 2020 / Eye proteome / EyeOme / Human Proteome Project See accompanying articles in the EyeOme series: http://dx.doi.org/10.1002/pmic.201600229; http://dx.doi.org/10.1002/pmic.201500188; http://dx.doi.org/10.1002/pmic.201400397 Proteomics has now published a series of four papers on compartments of the eye as shown in Fig. 1. As was noted [5], the human eye proteome [1–4]. Under the aegis of the Hu- it was not feasible to assess the quality of the data or estimate man Proteome Organization Biology and Disease-driven Hu- numbers of likely false positives in the heterogeneous studies man Proteome Project (HPP), the EyeOme was organized by from which these findings were summarized. -

Pupil Iris Ciliary Body
Eye iris pupil ciliary body Eyeball Anterior segment Posterior segment Pars caeca retinae Eyeball Corpus ciliare Procesus ciliares Sclera Iris Cornea Eyebulb wall Posterior Anterior segment segment Tunica externa Sclera Cornea (fibrosa) Tunica media Chorioidea Iris, Corpus (vasculosa) ciliare Tunica interna Pars optica Pars caeca (nervosa) retinae retinae Eyeball Fibrous tunic - tunica externa oculi • Cornea • Sclera limbus conjunctiva Cornea 1. Stratified squamous epithelium 2. Bowman´s membrane-anterior limiting lamina 3. Substantia propria cornae − 200 - 250 layers of regularly organized collagen fibrils − fibrocytes /keratocytes/ 4. Descemet´s membrane-posterior limiting lamina − the basement membrane of the posterior endothelium − Posterior endothelium − simple squamous epithelium Cornea Vascular tunic - tunica media oculi - Choroid ch - loose c.t. with network of blood vessels, numerous pigment cells c - Ciliary body - loose c.t. with smooth muscle cells – musculus i ciliaris /accomodation/ - ciliary processes – generate aqueous humor - Iris - central opening of the iris - the pupil Choroid 1. Lamina suprachoroidea /lamina fusca sclerae/ 2. Lamina vasculosa 3. Lamina chorocapillaris 4. Lamina vitrea /Bruch´s membrane/ L. suprachoroidea - perichoroidal space with melanocytes, collagen and elastic sclera fibers, fibroblasts, macrophages, lymphocytes L. vasculosa – blood supply – parallelní veins – c. ciliare choroid L. chorocapilaris – capillary plexus for retina L. vitrea – five layers, including balsal retina lamina of chorid endothelium and retinal pigment epithelium, collagen and elastic fibers. Choroid Ciliary body - structure ciliary processes m. ciliaris ciliary epithelium - outer cell layer is pigmented, inner cell layer is nonpigmented (pars caeca retinae) Ciliary body Epithelium two layers – basal, pigmented (fuscin), surface w/o pigment - Continuous with optical part of retina = pars caeca retinae processus ciliares m. -

Ciliary Body
Ciliary body S.Karmakar HOD Introduction • Ciliary body is the middle part of the uveal tract . It is a ring (slightly eccentric ) shaped structure which projects posteriorly from the scleral spur, with a meridional width varying from 5.5 to 6.5 mm. • It is brown in colour due to melanin pigment. Anteriorly it is confluent with the periphery of the iris (iris root) and anterior part of the ciliary body bounds a part of the anterior chamber angle. Introduction • Posteriorly ciliary body has a crenated or scalloped periphery, known as ora serrata, where it is continuous with the choroid and retina. The ora serrata exhibits forward extensions,known as dentate process, which are well defined on the nasal side and less so temporally. • Ciliary body has a width of approximately 5.9 mm on the nasal side and 6.7 mm on the temporal side. Extension of the ciliary body On the outside of the eyeball, the ciliary body extends from a point about 1.5 mm posterior to the corneal limbus to a point 6.5 to 7.5 mm posterior to this point on the temporal side and 6.5 mm posterior on the nasal side. Parts of ciliary body • Ciliary body, in cross section, is a triangular structure ( in diagram it can be compared as ∆ AOI). Outer side of the triangle (O) is attached with the sclera with suprachoroidal space in between. Anterior side of the triangle (A) forms part of the anterior & posterior chamber. In its middle, the iris is attached. The inner side of the triangle (I) is divided into two parts. -

Eye External Anatomy of Eye Accessory Structures
4/22/16 Eye Bio 40B Dr. Kandula External Anatomy of Eye Accessory Structures l Eyebrows l Levator Palpebrae Superioris - opens eye l Eyelashes l Ciliary glands – modified sweat glands l Small sebaceous glands l Sty is inflamed ciliary glands or small sebaceous glands 1 4/22/16 Terms: Lacrimal gland and duct Surface of eye Lacrimal puncta Lacrimal sac Nasolacrimal duct Nasal cavity Tears / Lacrimal fluid l a watery physiologic saline, with a plasma-like consistency, l contains the bactericidal enzyme lysozyme; l it moistens the conjunctiva and cornea, l provides nutrients and dissolved O2 to the cornea. Extrinsic Muscles of the Eye: Lateral/medial rectus Important to know Superior/inferior rectus actions and nerve Superior/inferior oblique supply in table 2 4/22/16 Extrinsic Eye Muscles • Eye movements controlled by six extrinsic eye muscles Four recti muscles § Superior rectus – moves eyeball superiorly supplied by Cranial Nerve III § Inferior rectus - moves eyeball inferiorly supplied by Cranial Nerve III § Lateral rectus - moves eyeball laterally supplied by Cranial Nerve VI § Medial rectus - moves eyeball medially supplied by Cranial Nerve III Extrinsic Eye Muscles Two oblique muscles rotate eyeball on its axis § Superior oblique rotates eyeball inferiorly and laterally and is supplied by Cranial Nerve IV § Inferior oblique rotates superiorly and laterally and is supplied by Cranial Nerve III Convergence of the Eyes l Binocular vision in humans has both eyes looking at the same object l As you look at an object close to your face, -

Anatomy & Physiology of The
Anatomy & Physiology of The Eye 2017-2018 Done By: 433 Team Abdullah M. Khattab Important Doctor’s Notes Extra Abdullah AlOmair Resources: Team 433, Doctors Notes, Vaughan & Asbury’s General ophthalmology. Editing File Embryology of The Eye ............................................................................................. 2 ● Defects: ........................................................................................................................... 2 Development of The Eye After Birth .......................................................................... 3 ● Refractive power depends on two factors: ...................................................................... 3 The Orbit ................................................................................................................... 4 ● Seven bones contribute the bony orbit and surrounded by nasal sinuses. .................... 4 ● The orbital wall, pear-like shaped, formed by: ................................................................ 4 ● Structures Passing Through the Optic Openings: ........................................................... 4 Extraocular Muscles .................................................................................................. 1 ● Anatomy .......................................................................................................................... 1 ● Notes: .............................................................................................................................. 1 ● Field of action: -

Form. They Are Probably Caused the Plasmodium. the Choroid, Ciliary
is thoroughly established, quinin may be discontinued packed together and contain no pigment. The lam- and tinctura cinchona? compound given instead. ina vitrea forms the inner coat of the choroid; it is Continued fevers, that are not typhoid, are often also devoid of pigment cells. The framework uniting encountered in malarial districts, over which quinin the vessels of the choroid is composed of delicate con- seems to exercise no influence. They can not be clas- nective tissue. sified as "irregular" malarial fevers for this reason, The arterial vessels entering into the formation of and because an examination of the blood does reveal the choroid are the short posterior ciliary arteries. the plasmodium, either in its regular or irregular These are about twenty in number and are derived (sestivo-autumnal) form. They are probably caused from the ophthalmic artery. They pass through the by an organism different from but closely related to sclera in a whorl or circle, close to the optic nerve, the plasmodium. dividing into branches and passing forward for some For the enlarged spleen but little can be done by little distance, where they change their course medication. Time will help the matter somewhat. obliquely inward, terminating in the capillary layer. For chronic malarial poisoning a change of resi- The venous system lies on the outside of the arteries dence, either temporary or permanent, to a colder and is arranged in curves (vasa vorticosa). They climate is demanded. unite, forming four or five principal trunks, which pass through the sclera about half way between the corneo-scleral and the nerve. -

Forward and Inward Movement of the Ciliary Muscle Apex with Accommodation in Adults
Forward and Inward Movement of the Ciliary Muscle Apex with Accommodation in Adults THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Trang Pham Prosak Graduate Program in Vision Science The Ohio State University 2014 Master's Examination Committee: Melissa D. Bailey, OD, PhD, Advisor Donald O. Mutti, OD, PhD Marjean Kulp, OD, PhD Copyright by Trang Pham Prosak 2014 Abstract Purpose: to study the inward and forward movement of the ciliary muscle during accommodation and to investigate the effects of one hour of reading on the ciliary muscle behavior in young adults. Methods: Subjects included 23 young adults with a mean age of 23.7 ± 1.9 years. Images of the temporal ciliary muscle of the right eye were obtained using the Visante™ Anterior Segment Ocular Coherence Tomography while accommodative response was monitored simultaneously by the Power-Refractor. Four images were taken at each accommodative response level (0, 4.0 and 6.0 D) before and after one hour of reading. Ciliary muscle thickness was measured at every 0.25 mm posterior to the scleral spur. SSMAX, which is the distance between scleral spur and the thickest point of the muscle (CMTMAX), was also measured. The change in the ciliary muscle thickness and SSMAX with accommodation from 0 to 4.0 D and 0 to 6.0 D was calculated. Paired t-tests were used to determine if the ciliary muscle thickness and SSMAX for the 4.0 and 6.0 diopters of accommodative response were different after one hour of reading. -

Anatomy of the Globe 09 Hermann D. Schubert Basic and Clinical
Anatomy of the Globe 09 Hermann D. Schubert Basic and Clinical Science Course, AAO 2008-2009, Section 2, Chapter 2, pp 43-92. The globe is the home of the retina (part of the embryonic forebrain, i.e.neural ectoderm and neural crest) which it protects, nourishes, moves or holds in proper position. The retinal ganglion cells (second neurons of the visual pathway) have axons which form the optic nerve (a brain tract) and which connect to the lateral geniculate body of the brain (third neurons of the visual pathway with axons to cerebral cortex). The transparent media of the eye are: tear film, cornea, aqueous, lens, vitreous, internal limiting membrane and inner retina. Intraocular pressure is the pressure of the aqueous and vitreous compartment. The aqueous compartment is comprised of anterior(200ul) and posterior chamber(60ul). Aqueous and vitreous compartments communicate across the anterior cortical gel of the vitreous which seen from up front looks like a donut and is called the “annular diffusional gap.” The globe consists of two superimposed spheres, the corneal radius measuring 8mm and the scleral radius 12mm. The superimposition creates an external scleral sulcus, the outflow channels anterior to the scleral spur fill the internal scleral sulcus. Three layers or ocular coats are distinguished: the corneal scleral coat, the uvea and neural retina consisting of retina and pigmentedepithelium. The coats and components of the inner eye are held in place by intraocular pressure, scleral rigidity and mechanical attachments between the layers. The corneoscleral coat consists of cornea, sclera, lamina cribrosa and optic nerve sheath. -

Morphometric Study of the Ciliary Ganglion and Its Pertinent Intraorbital Procedure L
Folia Morphol. Vol. 79, No. 3, pp. 438–444 DOI: 10.5603/FM.a2019.0112 O R I G I N A L A R T I C L E Copyright © 2020 Via Medica ISSN 0015–5659 journals.viamedica.pl Morphometric study of the ciliary ganglion and its pertinent intraorbital procedure L. Tesapirat1, S. Jariyakosol2, 3, V. Chentanez1 1Department of Anatomy, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand 2Department of Ophthalmology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand 3Ophthalmology Department, King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand [Received: 20 September 2019; Accepted: 12 October 2019] Background: Ciliary ganglion (CG) can be easily injured without notice in many intraorbital procedures. Surgical procedures approaching the lateral side of the orbit are at risk of CG injury which results in transient mydriasis and tonic pupil. This study aims to focus on the morphometric study of the CG which is pertinent to intraoperative procedure. Materials and methods: Forty embalmed cadaveric globes were dissected to ob- serve the location, shape and size of CG, characteristics and number of roots reaching CG, number of short ciliary nerve in the orbit. Distances from CG to posterior end of globe, optic nerve, lateral rectus muscle and its scleral insertion were measured. Results: Ciliary ganglion was located between optic nerve and lateral rectus in every case. Its shape could be oval, round and irregular. Mean width of CG was 2.24 mm and mean length was 3.50 mm. Concerning the roots, all 3 roots were present in 29 (72.5%) cases. Absence of motor root was found in 7 (17.5%) cases. -
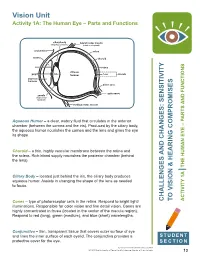
Vision Unit Activity 1A: the Human Eye – Parts and Functions
Vision Unit Activity 1A: The Human Eye – Parts and Functions ciliary body lateral rectus muscle (ring-shaped muscle) (rotation of eyeball) conjunctiva sclera cornea choroid iris retina vitreous pupil lens humour fovea macula aqueous humour blind spot optic nerve zonula (suspensory ligament) medical rectus muscle Aqueous Humor – a clear, watery fluid that circulates in the anterior chamber (between the cornea and the iris). Produced by the ciliary body, the aqueous humor nourishes the cornea and the lens and gives the eye its shape. Choroid – a thin, highly vascular membrane between the retina and the sclera. Rich blood supply nourishes the posterior chamber (behind the lens). Ciliary Body – located just behind the iris, the ciliary body produces aqueous humor. Assists in changing the shape of the lens as needed to focus. Cones – type of photoreceptor cells in the retina. Respond to bright light/ AND CHANGES: SENSITIVITY CHALLENGES VISION & HEARING COMPROMISES TO AND FUNCTIONS THE HUMAN EYE – PARTS 1A ACTIVITY illuminations. Responsible for color vision and fine detail vision. Cones are highly concentrated in fovea (located in the center of the macula region). Respond to red (long), green (medium), and blue (short) wavelengths. Conjunctiva – thin, transparent tissue that covers outer surface of eye and lines the inner surface of each eyelid. The conjunctiva provides a STUDENT protective cover for the eye. SECTION Teacher Enrichment Initiatives/CAINE 2013 © The University of Texas Health Science Center at San Antonio 13 Eyelid – Moveable fold of skin over the eye with lashes and glands along its margin. Eyelids provide protection from the environment, injury, and light.