ISCEV Guide to Visual Electrodiagnostic Procedures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blue Cone Monochromacy: Visual Function and Efficacy Outcome Measures for Clinical Trials
RESEARCH ARTICLE Blue Cone Monochromacy: Visual Function and Efficacy Outcome Measures for Clinical Trials Xunda Luo1☯‡, Artur V. Cideciyan1☯‡*, Alessandro Iannaccone2, Alejandro J. Roman1, Lauren C. Ditta2, Barbara J. Jennings2, Svetlana A. Yatsenko3, Rebecca Sheplock1, Alexander Sumaroka1, Malgorzata Swider1, Sharon B. Schwartz1, Bernd Wissinger4, Susanne Kohl4, Samuel G. Jacobson1* 1 Scheie Eye Institute, Department of Ophthalmology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America, 2 Hamilton Eye Institute, Department of Ophthalmology, University of Tennessee Health Science Center, Memphis, Tennessee, United States of America, 3 Pittsburgh Cytogenetics Laboratory, Center for Medical Genetics and Genomics, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, United States of America, 4 Molecular Genetics Laboratory, Institute for Ophthalmic Research, Centre for Ophthalmology, University of Tuebingen, Tuebingen, Germany ☯ These authors contributed equally to this work. ‡ OPEN ACCESS These authors are joint first authors on this work. * [email protected] (SGJ); [email protected] (AVC) Citation: Luo X, Cideciyan AV, Iannaccone A, Roman AJ, Ditta LC, Jennings BJ, et al. (2015) Blue Cone Monochromacy: Visual Function and Efficacy Abstract Outcome Measures for Clinical Trials. PLoS ONE 10(4): e0125700. doi:10.1371/journal.pone.0125700 Academic Editor: Dror Sharon, Hadassah-Hebrew University Medical Center, ISRAEL Background Blue Cone Monochromacy (BCM) is an X-linked retinopathy caused by mutations in the Received: December 29, 2014 OPN1LW / OPN1MW gene cluster, encoding long (L)- and middle (M)-wavelength sensitive Accepted: March 21, 2015 cone opsins. Recent evidence shows sufficient structural integrity of cone photoreceptors in Published: April 24, 2015 BCM to warrant consideration of a gene therapy approach to the disease. -

Updates on Myopia
Updates on Myopia A Clinical Perspective Marcus Ang Tien Y. Wong Editors Updates on Myopia Marcus Ang • Tien Y. Wong Editors Updates on Myopia A Clinical Perspective Editors Marcus Ang Tien Y. Wong Singapore National Eye Center Singapore National Eye Center Duke-NUS Medical School Duke-NUS Medical School National University of Singapore National University of Singapore Singapore Singapore This book is an open access publication. ISBN 978-981-13-8490-5 ISBN 978-981-13-8491-2 (eBook) https://doi.org/10.1007/978-981-13-8491-2 © The Editor(s) (if applicable) and The Author(s) 2020, corrected publication 2020 Open Access This book is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specifc statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. -

Colour Vision Deficiency
Eye (2010) 24, 747–755 & 2010 Macmillan Publishers Limited All rights reserved 0950-222X/10 $32.00 www.nature.com/eye Colour vision MP Simunovic REVIEW deficiency Abstract effective "treatment" of colour vision deficiency: whilst it has been suggested that tinted lenses Colour vision deficiency is one of the could offer a means of enabling those with commonest disorders of vision and can be colour vision deficiency to make spectral divided into congenital and acquired forms. discriminations that would normally elude Congenital colour vision deficiency affects as them, clinical trials of such lenses have been many as 8% of males and 0.5% of femalesFthe largely disappointing. Recent developments in difference in prevalence reflects the fact that molecular genetics have enabled us to not only the commonest forms of congenital colour understand more completely the genetic basis of vision deficiency are inherited in an X-linked colour vision deficiency, they have opened the recessive manner. Until relatively recently, our possibility of gene therapy. The application of understanding of the pathophysiological basis gene therapy to animal models of colour vision of colour vision deficiency largely rested on deficiency has shown dramatic results; behavioural data; however, modern molecular furthermore, it has provided interesting insights genetic techniques have helped to elucidate its into the plasticity of the visual system with mechanisms. respect to extracting information about the The current management of congenital spectral composition of the visual scene. colour vision deficiency lies chiefly in appropriate counselling (including career counselling). Although visual aids may Materials and methods be of benefit to those with colour vision deficiency when performing certain tasks, the This article was prepared by performing a evidence suggests that they do not enable primary search of Pubmed for articles on wearers to obtain normal colour ‘colo(u)r vision deficiency’ and ‘colo(u)r discrimination. -

Clinical and Genetic Investigation of a Large Tunisian Family with Complete Achromatopsia: Identification of a New Nonsense Mutation in GNAT2 Gene
Journal of Human Genetics (2011) 56, 22–28 & 2011 The Japan Society of Human Genetics All rights reserved 1434-5161/11 $32.00 www.nature.com/jhg ORIGINAL ARTICLE Clinical and genetic investigation of a large Tunisian family with complete achromatopsia: identification of a new nonsense mutation in GNAT2 gene Farah Ouechtati1,2,7, Ahlem Merdassi2,7, Yosra Bouyacoub1,2, Leila Largueche2, Kaouther Derouiche2, Houyem Ouragini1, Sonia Nouira1, Leila Tiab3,4, Karim Baklouti2, Ahmed Rebai5, Daniel F Schorderet3,4,6, Francis L Munier3,4,6, Leonidas Zografos4,6, Sonia Abdelhak1 and Leila El Matri2 Complete achromatopsia is a rare autosomal recessive disease associated with CNGA3, CNGB3, GNAT2 and PDE6C mutations. This retinal disorder is characterized by complete loss of color discrimination due to the absence or alteration of the cones function. The purpose of the present study was the clinical and the genetic characterization of achromatopsia in a large consanguineous Tunisian family. Ophthalmic evaluation included a full clinical examination, color vision testing and electroretinography. Linkage analysis using microsatellite markers flanking CNGA3, CNGB3, GNAT2 and PDE6C genes was performed. Mutations were screened by direct sequencing. A total of 12 individuals were diagnosed with congenital complete achromatopsia. They are members of six nuclear consanguineous families belonging to the same large consanguineous family. Linkage analysis revealed linkage to GNAT2. Mutational screening of GNAT2 revealed three intronic variations c.119À69G4C, c.161+66A4T and c.875À31G4C that co-segregated with a novel mutation p.R313X. An identical GNAT2 haplotype segregating with this mutation was identified, indicating a founder mutation. All patients were homozygous for the p.R313X mutation. -
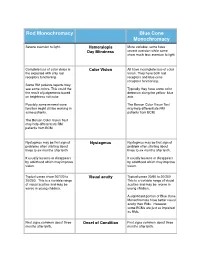
Comparison RM Versus BCM 2
Rod Monochromacy Blue Cone Monochromacy Severe aversion to light. Hemeralopia More variable- some have Day Blindness severe aversion while some show much less aversion to light. Complete loss of color vision is Color Vision All have incomplete loss of color the expected with only rod vision. They have both rod receptors functioning. receptors and blue cone receptors functioning. Some RM patients reports they see some colors. This could the Typically they have some color the result of judgements based detection along the yellow- blue on brightness not color. axis. Possibly some minimal cone The Berson Color Vision Test function might still be working in may help differentiate RM some patients. patients from BCM. The Berson Color Vision Test may help differentiate RM patients from BCM. Nystagmus may be first sign of Nystagmus Nystagmus may be first sign of problems often starting about problem often starting about three to six months after birth. three to six months after birth. It usually lessens or disappears It usually lessens or disappears by adulthood which may improve by adulthood which may improve vision. vision. Typical cases show 20/120 to Visual acuity Typical cases 20/60 to 20/200 20/200. This is a variable range This is a variable range of visual of visual acuities and may be acuities and may be worse in worse in young children. young children. A significant portion of Blue Cone Monochromats have better visual acuity than RMs. However, some BCMs are just as impaired as RMs. First signs common about three Onset of Condition First signs common about three months after birth. -

Visual Impairment Age-Related Macular
VISUAL IMPAIRMENT AGE-RELATED MACULAR DEGENERATION Macular degeneration is a medical condition predominantly found in young children in which the center of the inner lining of the eye, known as the macula area of the retina, suffers thickening, atrophy, and in some cases, watering. This can result in loss of side vision, which entails inability to see coarse details, to read, or to recognize faces. According to the American Academy of Ophthalmology, it is the leading cause of central vision loss (blindness) in the United States today for those under the age of twenty years. Although some macular dystrophies that affect younger individuals are sometimes referred to as macular degeneration, the term generally refers to age-related macular degeneration (AMD or ARMD). Age-related macular degeneration begins with characteristic yellow deposits in the macula (central area of the retina which provides detailed central vision, called fovea) called drusen between the retinal pigment epithelium and the underlying choroid. Most people with these early changes (referred to as age-related maculopathy) have good vision. People with drusen can go on to develop advanced AMD. The risk is considerably higher when the drusen are large and numerous and associated with disturbance in the pigmented cell layer under the macula. Recent research suggests that large and soft drusen are related to elevated cholesterol deposits and may respond to cholesterol lowering agents or the Rheo Procedure. Advanced AMD, which is responsible for profound vision loss, has two forms: dry and wet. Central geographic atrophy, the dry form of advanced AMD, results from atrophy to the retinal pigment epithelial layer below the retina, which causes vision loss through loss of photoreceptors (rods and cones) in the central part of the eye. -

The Genetics of Myopia 5 Milly S
The Genetics of Myopia 5 Milly S. Tedja, Annechien E. G. Haarman, Magda A. Meester-Smoor, Virginie J. M. Verhoeven, Caroline C. W. Klaver, and Stuart MacGregor Key Points • While the recent global rise of myopia prevalence is primarily attributable to environmental changes, within populations inherited factors play a large role in explaining why some individuals are affected by myopia while oth- ers are not. • Early efforts to identify the specific genes underlying the heritability of refractive error used linkage and candidate gene designs to identify up to 50 loci and genes, although most remain unconfirmed. M. S. Tedja · A. E. G. Haarman · M. A. Meester-Smoor Department of Ophthalmology, Erasmus Medical Center, Rotterdam, The Netherlands Department of Epidemiology, Erasmus Medical Center, Rotterdam, The Netherlands V. J. M. Verhoeven Department of Ophthalmology, Erasmus Medical Center, Rotterdam, The Netherlands Department of Epidemiology, Erasmus Medical Center, Rotterdam, The Netherlands Department of Clinical Genetics, Erasmus Medical Center, Rotterdam, The Netherlands C. C. W. Klaver Department of Ophthalmology, Erasmus Medical Center, Rotterdam, The Netherlands Department of Epidemiology, Erasmus Medical Center, Rotterdam, The Netherlands Department of Ophthalmology, Radboud University Medical Center, Nijmegen, The Netherlands S. MacGregor (*) Statistical Genetics, QIMR Berghofer Medical Research Institute, Brisbane, QLD, Australia e-mail: [email protected] © The Author(s) 2020 95 M. Ang, T. Y. Wong (eds.), Updates on Myopia, https://doi.org/10.1007/978-981-13-8491-2_5 96 M. S. Tedja et al. • As the sample size in genome-wide association studies (GWAS) has increased, the number of implicated loci has risen steadily, with 161 vari- ants reported in the latest meta-analysis. -

Causes of Color Blindness: Function and Failure of the Genes That Detect Color
A REVIEW ON COLOR BLINDNESS 1 Causes of Color Blindness: Function and Failure of the Genes that Detect Color Dylan Taylor A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Fall 2020 A REVIEW ON COLOR BLINDNESS 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ Gary D. Isaacs Jr., Ph.D. Thesis Chair ______________________________ Michael S. Price, Ph.D. Committee Member _____________________________ James H. Nutter, D.A. Honors Director ______________________________ Date A REVIEW ON COLOR BLINDNESS 3 Abstract Color blindness affects nearly 10% of the entire population, with multiple types of color blindness from various genetic mutations. In the following sections, the nature of light and how the human eye perceives light will be discussed. Afterward, the major forms of color blindness and their genetic causes will be considered. Once these genetic causes have been established, the current method for diagnosing color blindness will be investigated, followed by a discussion of the current treatments available to those with color blindness. Finally, a brief discussion will address possible future work for color blindness with the hope of finding better treatments and a future prevention. A REVIEW ON COLOR BLINDNESS 4 Causes of Color Blindness: Function and Failure of the Genes that Detect Color Introduction Without the human eye’s ability to detect color, the world would appear as dull as a black and white movie. However, despite our ability to detect color, not all humans perceive the same colors as one another. -

The Alter Retina: Alternative Splicing of Retinal Genes in Health and Disease
International Journal of Molecular Sciences Review The Alter Retina: Alternative Splicing of Retinal Genes in Health and Disease Izarbe Aísa-Marín 1,2 , Rocío García-Arroyo 1,3 , Serena Mirra 1,2 and Gemma Marfany 1,2,3,* 1 Departament of Genetics, Microbiology and Statistics, Avda. Diagonal 643, Universitat de Barcelona, 08028 Barcelona, Spain; [email protected] (I.A.-M.); [email protected] (R.G.-A.); [email protected] (S.M.) 2 Centro de Investigación Biomédica en Red Enfermedades Raras (CIBERER), Instituto de Salud Carlos III (ISCIII), Universitat de Barcelona, 08028 Barcelona, Spain 3 Institute of Biomedicine (IBUB, IBUB-IRSJD), Universitat de Barcelona, 08028 Barcelona, Spain * Correspondence: [email protected] Abstract: Alternative splicing of mRNA is an essential mechanism to regulate and increase the diversity of the transcriptome and proteome. Alternative splicing frequently occurs in a tissue- or time-specific manner, contributing to differential gene expression between cell types during development. Neural tissues present extremely complex splicing programs and display the highest number of alternative splicing events. As an extension of the central nervous system, the retina constitutes an excellent system to illustrate the high diversity of neural transcripts. The retina expresses retinal specific splicing factors and produces a large number of alternative transcripts, including exclusive tissue-specific exons, which require an exquisite regulation. In fact, a current challenge in the genetic diagnosis of inherited retinal diseases stems from the lack of information regarding alternative splicing of retinal genes, as a considerable percentage of mutations alter splicing Citation: Aísa-Marín, I.; or the relative production of alternative transcripts. Modulation of alternative splicing in the retina García-Arroyo, R.; Mirra, S.; Marfany, is also instrumental in the design of novel therapeutic approaches for retinal dystrophies, since it G. -

CTA's Best Practices for Colour Blind Friendly Publications And
BEST PRACTICES FOR COLOUR BLIND FRIENDLY PUBLICATIONS & DESCRIPTIONS CTAO Astrodiversity Project | Last update: October 2020 Prepared by: Alba Fernández-Barral Outreach & Education Coordinator of the CTA Observatory (CTAO) 1 Table of Contents I. Introduction and Guidelines ................................................................................................................. 3 II. Support Material ...................................................................................................................................... 6 III. References ................................................................................................................................................. 6 IV. Contact ........................................................................................................................................................ 6 2 I. Introduction and Guidelines Colour blindness (or colour vision deficiency, CVD) refers to a reduced capacity or complete incapacity to distinguish colours and affects approximately one in 12 men and one in 200 women [1]. The most common type of CVD is the red-green colour blindness, which despite its name, does not only affect the visibility of red and green but the whole spectrum of colours, as red and green are part of every other colour to some extent. There are different types of CVD (Figure 1): Protanomaly (red-weakness) or Protanopia (red-blindness), Deuteranomaly (green-weakness) or Deuteranopia (green-blindness), as well as Tritanomaly (blue-weakness) or Tritanopia -

BCM&School-EN-Final 3
Board of Directors BEST PRACTICES FOR SCHOOL INTEGRATION OF President STUDENTS WITH BLUE CONE MONOCHROMACY Renata Sarno, Ph.D. (BCM) Secretary Kay McCrary, Ed.D. By BCM Families Foundation - October 2015 Treasurer Barbara Sergent, MBA This document contains notes and advice collected during many years Scientific Advisory Board of firsthand experience by men who have BCM and by families with children who have BCM, most of whom live in USA and in Europe. It Jeremy Nathans, MD, Ph.D. has been written for teachers of Primary and Secondary/Elementary, The Johns Hopkins School of Middle and High Schools. We hope this document will help students Medicine, Baltimore, Maryland with BCM by giving their teachers useful information about the visual Samuel G. Jacobson, MD, Ph.D. impairments caused by BCM, plus by recommending proven strategies Scheie Eye Institute, University of to better integrate the BCM student. Philadelphia William W. Hauswirth, Ph.D. University of Florida Thanks to all the people contributing to editing this document: Kay John G. Flannery, Ph.D. McCrary Ed.D., Renata Sarno, Ph.D., Trudi Dawson, Valentina Della Volpe University of California, Berkeley Ph.D, and to all the families who sent us advice and comments. We thank Dr. Laura K. Windsor of the Low Vision Center of Indiana, because her Alessandro Iannaccone, MD,M.S. Hamilton Eye Institute,University Teacher’s Guide for Achromatopsia inspired us to write this guide for of Tennessee BCM. Bernd Wissinger, Ph.D. University of Tuebingen, Germany Communication or comments can be sent to: [email protected] – new suggestions are welcomed! Toward the cure of Blue Cone Monochromacy BCM Families Foundation PO Box 7711 Jupiter, FL 33458-7711 USA [email protected] BCM Families Foundation is recognized as a non-profit organization by the Internal Revenue www.BCMFamilies.org Service. -

COLOR BLINDNESS What Does It Look Like?
COLOR BLINDNESS What Does It Look Like? Ever wondered what the world looks like to people with color blindness? Curious minds often wish to see through the eyes of others. Check out the photos below to better understand and empathize with fellow travelers in life. Normal Vision As designers, we have special interest in color blindness — more correctly called “Color Vision Deficiency” and more properly called Dyschromatopsia — as we work daily with colors. We can better provide for those with color vision deficiencies by taking it into consideration as we design our websites, ads, illustrations, logos, typography or any visual communications. Protanomaly Protanopia Photos Show Vision Types Photos below show types of color blindness accessed on the ColorJack “Vision” menu: The less severe type, Anomalous Trichromacy, includes: • Protanomaly - reds appear weak • Deuteranomaly - greens appear weak • Tritanomaly - very rare, blues appear weak Deuteranomaly Deuteranopia The moderately severe type, Dichromacy, includes: • Protanopia: reds are greatly reduced • Deuteranopia - greens are greatly reduced • Tritanopia - rare; blues are greatly reduced The most severe type, Monochromacy, is very rare: all, or most, color vision is lacking: Rod Monochromacy (Achromatopsia) and Achromatomaly. The “Customaly” option offers the opportunity to Tritanomaly Tritanopia create your own custom vision deficiency! :o) Causes of Color Blindness Color blindness is usually passed on at birth, but can come about through illnesses or accidents. Causes are generally related to the eye’s retinal cones being defective or absent. Explore deeper into color blindness at Wikipedia, U of I Eye Center, and Health A to Z. —BD Achromatomaly Achromatopsia.