Use of an Electrolyte for Driving Electrolytic Capacitor and Electrolytic Capacitor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wednesday May 26, 1999
5±26±99 Vol. 64 No. 101 Wednesday Pages 28333±28712 May 26, 1999 federal register 1 VerDate 06-MAY-99 21:29 May 25, 1999 Jkt 183247 PO 00000 Frm 00001 Fmt 4710 Sfmt 4710 E:\FR\FM\26MYWS.XXX pfrm03 PsN: 26MYWS II Federal Register / Vol. 64, No. 101 / Wednesday, May 26, 1999 The FEDERAL REGISTER is published daily, Monday through SUBSCRIPTIONS AND COPIES Friday, except official holidays, by the Office of the Federal Register, National Archives and Records Administration, PUBLIC Washington, DC 20408, under the Federal Register Act (44 U.S.C. Subscriptions: Ch. 15) and the regulations of the Administrative Committee of Paper or fiche 202±512±1800 the Federal Register (1 CFR Ch. I). The Superintendent of Assistance with public subscriptions 512±1806 Documents, U.S. Government Printing Office, Washington, DC 20402 is the exclusive distributor of the official edition. General online information 202±512±1530; 1±888±293±6498 Single copies/back copies: The Federal Register provides a uniform system for making available to the public regulations and legal notices issued by Paper or fiche 512±1800 Federal agencies. These include Presidential proclamations and Assistance with public single copies 512±1803 Executive Orders, Federal agency documents having general FEDERAL AGENCIES applicability and legal effect, documents required to be published Subscriptions: by act of Congress, and other Federal agency documents of public Paper or fiche 523±5243 interest. Assistance with Federal agency subscriptions 523±5243 Documents are on file for public inspection in the Office of the Federal Register the day before they are published, unless the issuing agency requests earlier filing. -

Loba Chemie Pvt
TABLE OF CONTENT INTRODUCTION Contact US ii MD Letter iii Company Profile iv Latest Information v Label vi Certifications vii GMP Compliant Pharma Facility viii QC Capability ix R&D x Logistic xi COA xii SDS xiii Packing xiv Quality xvi Terms Of Sales xvii Application xviii Product Highlights xix Nanopowder & Carbon Nanotubes xxvii List of New Products xxviii ALPHABETICAL PRODUCT LISTING Price List Chemical 01-155 Ecosafe Safety Products 156-160 Macherey-Nagel Filtration Products 161-181 [email protected] I CONTACT US Contact us for more information on any of our products and services HEAD OFFICE - MUMBAI Loba Chemie Pvt. Ltd., Jehangir Villa, 107, Wode House Road, Colaba, Mumbai-400005 Maharashtra, India Tel: +91(022) 6663 6663 Fax: +91(022) 6663 6699 MANUFACTURING UNIT (TARAPUR) Loba Chemie Pvt. Ltd., Plot No.: D-22, M.I.D.C. Tarapur, Boisar, Taluka: Palghar, Thane-401506 Maharashtra, India Ph: +91(02525) 300 001 Stay up to date about our range and availability www.lobachemie.com Get in touch with us General Enquiry: [email protected] Technical Query: [email protected] Domestic Sales: [email protected] Export Sales: [email protected] Follow us on: ii www.lobachemie.com WELCOME AT LOBA CHEMIE Dear Valued Reader Another exciting year has gone by and we would like to start the New Year, as usual, with a new catalogue featuring more innova- tive highest quality range of routine and novel Laboratory Chemi- cal and Fine Reagents. With our more than 4 decads of expertise in Laboratory Chemical and Fine Reagents we not only bring you a complete Laboratory at your fingertips but in addition with our expertise we have palced our brand of products in a very competitive position for years to come. -

Otto-Catalog-2019-20.Pdf
Lab Chemicals & More..... Otto Catalog 2019-20 1 CODE PRODUCT NAME CAS NO. PACKING RATE ` PACKING RATE ` A 1214 ABSCISIC ACID practical grade 10% 14375-45-2 100mg 2007 1gm 13059 A 1215 ABSCISIC ACID for biochemistry 99% 14375-45-2 25mg 1395 100mg 3609 500 mg 17469 A 1217 (7-AMINO CEPHALOSPORANIC ACID) 7-ACA 98% 957-68-6 1gm 2403 5gm 9396 A 1225 ACACIA 9000-01-5 500gm 504 5kg 4392 A 1226 ACACIA spray dried powder 9000-01-5 500gm 684 5kg 6309 A 1227 ACACIA GR 9000-01-5 500gm 828 5kg 7407 A 0855 ACARBOSE, >95% 56180-94-0 1 gm 18099 A 1229 ACENAPHTHENE pract 83-32-9 100gm 306 500gm 1395 5 kg 11907 A 1230 ACENAPHTHENE for synthesis 97% 83-32-9 100gm 450 500gm 1692 A 1231 ACENAPHTHENE GR for HLPC 83-32-9 100gm 1359 500gm 5533 A 1234 ACES BUFFER 99% 7365-82-4 5gm 864 25gm 2385 [N-(2-Acetamido)-2-aminoethane sulfonic acid] 100 gm 8739 A 1233 ACETALDEHYDE 20-30% solution for synthesis 75-07-0 500ml 477 5lt 4095 A 1235 ACETAMIDE for synthesis 99% 60-35-5 500 gm 801 A 1240 ACETAMIDINE CHLORIDE for synthesis 124-42-5 100gm 3159 250gm 7830 A 1242 N-(2-ACETAMIDO) IMINODIACETIC ACID (ADA BUFFER) 26239-55-4 25gm 855 100gm 2592 250 gm 5994 A 1245 ACETANILIDE for synthesis 98.5% 103-84-4 500gm 918 5kg 8289 A 1248 ACETATE BUFFER SOLUTION pH 4.6 - - - - - 500ml 180 5lt 1449 A 1250 ACETIC ACID glacial 99% 64-19-7 500ml 207 5lt 1602 A 1251 ACETIC ACID glacial GR 99%+ 64-19-7 500ml 252 5lt 1908 A 1252 ACETIC ACID GLACIAL GR 99.7% 64-19-7 500ml 315 5lt 1998 A 1253 ACETIC ACID GLACIAL EL 99.9% 64-19-7 500ml 378 5lt 2502 A 1254 ACETIC ACID 99.8% for HPLC 64-19-7 -

Phase of Atmospheric Secondary Organic Material Affects Its Reactivity
Phase of atmospheric secondary organic material affects its reactivity Mikinori Kuwata and Scot T. Martin1 School of Engineering and Applied Sciences and Department of Earth and Planetary Sciences, Harvard University, Cambridge, MA 02138 Edited by Mark H. Thiemens, University of California San Diego, La Jolla, CA, and approved September 11, 2012 (received for review May 29, 2012) The interconversion of atmospheric organic particles among solid, The atmospheric occurrence of organic semisolid particles can semisolid, and liquid phases is of keen current scientific interest, have important implications for aging processes. Aging can sig- especially for particles of secondary organic material (SOM). Here- nificantly alter a particle’s physicochemical properties, including in, the influence of phase on ammonia uptake and subsequent hygroscopic and optical properties (1). Diffusion of molecules particle-phase reactions was investigated for aerosol particles of in a semisolid, however, is much slower than in a liquid. The adipic acid and α-pinene ozonolysis SOM. The nitrogen content Stokes–Einstein equation states that the diffusion coefficient D of the particles was monitored by online mass spectrometry for is proportional to η−1 (12). The timescale for homogeneous mix- increasing ammonia exposure. Solid and semisolid adipic acid ing by diffusion is on the order of microseconds to milliseconds particles were inert to the ammonia uptake for low RH (<5%). for liquid submicron particles. By comparison, for semisolid par- For the solid particles, ammonia exposure at high relative humidity ticles the timescale can range from seconds to days, depending on (RH; >94%) induced a first-order deliquescence phase transition the viscosity of particles (16). -

Product List 2013-14 Cat
RANGE OF LABORATORY CHEMICALS PRODUCT LIST 2013-14 CAT. NOS. PRODUCT NAME CAS.NOS. PACKING A-00141 ABSCISIC ACID 99% (For Biochemistry) 21293-29-8 25 mg (ABA) 100 mg A-00151 ACACIA (Confirming to IP) 9000-01-05 500 gm (Gum Acacia Powder ) 10 x 500 gm A-00158 ACENAPHTHENE 96% (For Synthesis) 83-32-9 100 gm 500 gm A-00160 ACES BUFFER 99% 7365-82-4 25 gm [N-2-Acetamido-2- Aminoethane Sulphonic Acid] 100 gm A-00163 ACETALDEHYDE SOLUTION 20-30% 75-07-0 500 ml 2.5 Ltr. A-00168 ACETAMIDE 99% (For Synthesis) 60-35-5 500 gm A-00170 ACETAMIDINIUM HYDROCHLORIDE 124-42-5 100 gm (Acetamidium Chloride) A-00178 ACETANILIDE 98.5 % (For Synthesis) 103-84-4 500 gm (N-Phenyl Acetamide) 10 x 500 gm A-00180 ACETATE BUFFER SOLUTION PH 4.6 126-96-5 500 ml A-00188 ACETIC ACID GLACIAL (For Synthesis) 64-19-7 500 ml 2.5Ltr. ACETIC ACID GLACIAL 99.7% AR 64-19-7 500 ml A-00188/100 2.5 lit. A-00189 ACETIC ACID METHYLAMIDE 79-16-3 100 ml A-00190 ACETIC ACID SOLUTION 0.1N --------- 500 ml A-00191 ACETIC ACID SOLUTION 1%, 2%, 3% --------- 500ml A-00193 ACETO ACETANILIDE 98% (For Synthesis) 102-01-2 500 gm A-00199 O-ACETO ACETANISIDIDE 98% 92-15-9 100 gm A-00205 ACETO CARMINE SOLUTION --------- 100 ml (For Microscopical Staining) 500 ml A-00206 ACETO CARMINE SOLUTION AR --------- 100 ml 500 ml A-00210 ACETONE 99% (EX - PURE) 67-64-1 500 ml (For Synthesis) 25 x 500 ml 2.5Ltr. -

Salts Are Our Life Mg
Zn Fe Salts are our Life Mg Ca K www.lohmann4minerals.com The whole is more than the sum of its Parts. Aristoteles 2 Dr. Paul Lohmann® management f.l.t.r.: Dr. Uwe Günther, Torsten Cuno, Jürgen Lohmann Achieving more together The famous chemist Justus von Liebig said: “Salt is the most precious of all jewels which the earth gives us.” Mineral salts are essential to all life on our planet. For us at the Dr. Paul Lohmann® company, these salts are also vitally important—salts are our life! Dr. Paul Lohmann GmbH KG manufactures a broad portfolio of mineral salts for use in a variety of applications. We’ve been producing these special salts for over 130 years and of course have a great deal of experience. Based on this long-standing tradition we have the expertise to reliably develop and produce high-quality mineral salts for the pharma- ceutical industry, for food and nutritional supplements, cosmetics and many other areas in chemistry and technology. Tradition meets innovation. Mineral salts are exciting chemical compounds. Our developers design new innovative products, combinations and applications in collaboration with our customers. Our research, development and application center is continuously generating new ideas, developing a great deal of potential in many markets. At the Dr. Paul Lohmann® company, we gladly take on the challenges—worldwide. We are here to serve people—salts are our life! In 1886, chemist Dr. Paul Lohmann began 1886 producing reduced iron (ferrum reductum) for the treatment of iron defi ciency in a vacant factory located at Feuergraben in Hamelin. -

Volatility of Mixed Atmospheric Humic-Like Substances and Ammonium Sulfate Particles Wei Nie1,2,3,8*, Juan Hong3, Silja A. K. H
1 Volatility of mixed atmospheric Humic-like Substances and ammonium sulfate particles 2 Wei Nie1,2,3,8*, Juan Hong3, Silja A. K. Häme3, Aijun Ding1,2,8*, Yugen Li4, Chao Yan3, Liqing Hao5, Jyri 3 Mikkilä3, Longfei Zheng1,2,8, Yuning Xie1,2,8, Caijun Zhu1,2,8, Zheng Xu1,2,8, Xuguang Chi1,2,8, Xin 4 Huang1,2,8, Yang Zhou6,7, Peng Lin6,a, Annele Virtanen5, Douglas R. Worsnop3, Markku Kulmala3, 5 Mikael Ehn3, Jianzhen Yu6, Veli-Matti Kerminen3 and Tuukka Petäjä3,1 6 1 Joint International Research Laboratory of Atmospheric and Earth System Sciences, Nanjing University, 7 Nanjing, China 8 2 Institute for Climate and Global Change Research & School of Atmospheric Sciences, Nanjing University, 9 Nanjing, 210023, China 10 3 Division of Atmospheric Sciences, Department of Physics, University of Helsinki, Helsinki, Finland 11 4 Division of Environment, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, 12 Hong Kong, China 13 5 Department of Applied Physics, University of Eastern Finland, Kuopio 70211, Finland 14 6 Department of Chemistry, Hong Kong University of Science & Technology, Clear Water Bay, Kowloon, Hong 15 Kong, China 16 7 Key Laboratory of Physical Oceanography, College of Oceanic and Atmospheric Sciences, Ocean University 17 of China, Qingdao 266100, China 18 8 Collaborative Innovation Center of Climate Change, Jiangsu province, China 19 a now at: Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, Richland, WA 20 99532 *Correspondence to: W. Nie ([email protected]) and A. J. Ding ([email protected]) 21 Abstract 22 The volatility of organic aerosols remains poorly understood due to the complexity of speciation and 23 multi-phase processes. -

Industrial Chemicals
Industrial Chemicals Art no. Product Name CAS no. Packing Prices in INR Prices in Dollar UN no. AL0001 ACACIA 9000 – 01– 5 5KG 3800 $84.44 1 AL0002 ACACIA (gum acacia powder) 9000 – 01– 5 500GM 435 $9.67 AL0003 ACACIA (enzyme free) AR 9000 – 01– 5 500GM 750 $16.67 AL0004 ACACIA SPRAY DRIED POWDER 9000 – 01– 5 500GM 375 $8.33 AL0005 ACENAPHTHENE 96% for synthesis 82-32-9 500GM 1080 $24 AL0006 ACENAPHTHENE 96% for synthesis 82-32-9 100GM 270 $6 AL0007 ACETALDEHYDE SOLUTION (20% - 30%) for synthesis 75-07-0 2.5LIT 1150 $25.56 1089 AL0008 ACETALDEHYDE SOLUTION (20% - 30%) for synthesis 75-07-0 500ML 240 $5.33 1089 AL0009 ACETAMIDE 99% FOR SYNTHESIS 60-35-5 500GM 480 $10.67 AL0010 ACETANILIDE 98.5% 103-84-4 500GM 540 $12 AL0011 ACETIC ACID FOR HPLC 99.8% 64-19-17 2.5LIT 1650 $36.67 2789 AL0012 ACETIC ACID FOR HPLC 99.8% 64-19-17 1LIT 700 $15.56 2789 AL0013 ACETIC ACID GLACIAL 99.5% 64-19-17 2.5LIT 645 $14.33 2789 AL0014 ACETIC ACID GLACIAL 99.5% 64-19-17 500ML 165 $3.67 2789 AL0015 ACETIC ACID GLACIAL EL 99.9% 64-19-17 2.5LIT 1200 $26.67 2789 AL0016 ACETIC ACID GLACIAL EL 99.9% 64-19-17 500ML 400 $8.89 2789 AL0017 ACETIC ACID GLACIAL 99.7% AR indifferent to chromic acid 64-19-17 2.5LIT 825 $18.33 2789 AL0018 ACETIC ACID GLACIAL 99.7% AR indifferent to chromic acid 64-19-17 500ML 210 $4.67 2789 AL0019 ACETIC ACID GLACIAL AR (ALDEHYDE FREE) 64-19-17 2.5LIT 970 $21.56 2789 AL0020 ACETIC ACID GLACIAL AR (ALDEHYDE FREE) 64-19-17 500ML 250 $5.56 2789 AL0021 ACETO ORCEIN (connective tissue stain) 100ML 320 $7.11 AL0022 ACETOACETANILIDE FOR SYNTHESIS -

Volatility of Mixed Atmospheric Humic-Like Substances and Ammonium Sulfate Particles
CORE Metadata, citation and similar papers at core.ac.uk Provided by Helsingin yliopiston digitaalinen arkisto Atmos. Chem. Phys., 17, 3659–3672, 2017 www.atmos-chem-phys.net/17/3659/2017/ doi:10.5194/acp-17-3659-2017 © Author(s) 2017. CC Attribution 3.0 License. Volatility of mixed atmospheric humic-like substances and ammonium sulfate particles Wei Nie1,2,3,8, Juan Hong3, Silja A. K. Häme3, Aijun Ding1,2,8, Yugen Li4, Chao Yan3, Liqing Hao5, Jyri Mikkilä3, Longfei Zheng1,2,8, Yuning Xie1,2,8, Caijun Zhu1,2,8, Zheng Xu1,2,8, Xuguang Chi1,2,8, Xin Huang1,2,8, Yang Zhou6,7, Peng Lin6,a, Annele Virtanen5, Douglas R. Worsnop3, Markku Kulmala3, Mikael Ehn3, Jianzhen Yu6, Veli-Matti Kerminen3, and Tuukka Petäjä3,1 1Joint International Research Laboratory of Atmospheric and Earth System Sciences, Nanjing University, Nanjing, China 2Institute for Climate and Global Change Research & School of Atmospheric Sciences, Nanjing University, Nanjing, 210023, China 3Division of Atmospheric Sciences, Department of Physics, University of Helsinki, Helsinki, Finland 4Division of Environment, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong SAR, China 5Department of Applied Physics, University of Eastern Finland, Kuopio 70211, Finland 6Department of Chemistry, Hong Kong University of Science & Technology, Clear Water Bay, Kowloon, Hong Kong SAR, China 7Key Laboratory of Physical Oceanography, College of Oceanic and Atmospheric Sciences, Ocean University of China, Qingdao 266100, China 8Collaborative Innovation Center of Climate Change, Jiangsu province, China anow at: Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, Richland, WA 99532, USA Correspondence to: Wei Nie ([email protected]) and Aijun Ding ([email protected]) Received: 20 September 2016 – Discussion started: 11 October 2016 Revised: 27 February 2017 – Accepted: 28 February 2017 – Published: 15 March 2017 Abstract. -
Qualikems Price List 2014-15
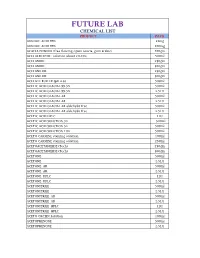
FUTURE LAB CHEMICAL LIST PRODUCT PACK ABSCISIC ACID 99% 25mg ABSCISIC ACID 99% 100mg ACACIA POWDER (free flowing) (gum acacia, gum arabic) 500gm ACETALDEHYDE solution about 20-30% 500ml ACETAMIDE 250gm ACETAMIDE 500gm ACETANILIDE 250gm ACETANILIDE 500gm ACETATE BUFFER (pH 4.6) 500ml ACETIC ACID GLACIAL 99.5% 500ml ACETIC ACID GLACIAL 99.5% 2.5Ltr ACETIC ACID GLACIAL AR 500ml ACETIC ACID GLACIAL AR 2.5Ltr ACETIC ACID GLACIAL AR aldehyde free 500ml ACETIC ACID GLACIAL AR aldehyde free 2.5Ltr ACETIC ACID HPLC 1Ltr ACETIC ACID SOLUTION 3% 500ml ACETIC ACID SOLUTION 5% 500ml ACETIC ACID SOLUTION 10% 500ml ACETO CARMINE staining solution 100ml ACETO CARMINE staining solution 250ml ACETOACETANILIDE (Tech) 250gm ACETOACETANILIDE (Tech) 500gm ACETONE 500ml ACETONE 2.5Ltr ACETONE AR 500ml ACETONE AR 2.5Ltr ACETONE HPLC 1Ltr ACETONE HPLC 2.5Ltr ACETONITRILE 500ml ACETONITRILE 2.5Ltr ACETONITRILE AR 500ml ACETONITRILE AR 2.5Ltr ACETONITRILE HPLC 1Ltr ACETONITRILE HPLC 2.5Ltr ACETO ORCEIN Solution 100ml ACETOPHENONE 500ml ACETOPHENONE 2.5Ltr ACETYLACETONE 250ml ACETYLACETONE 500ml ACETYLACETONE 2.5Ltr ACETYLACETONE AR 100ml ACETYLACETONE AR 500ml ACETYL BROMIDE 250ml ACETYL BROMIDE 500ml ACETYL CHLORIDE 500ml ACETYL CHLORIDE 2.5Ltr ACETYLCHOLINE CHLORIDE 25gm ACETYLCHOLINE CHLORIDE 100gm ACETYL SALICYLIC ACID 250gm ACETYL SALICYLIC ACID 500gm ACID ALCOHOL 125ml ACID ALCOHOL 500ml ACID MIXTURE (phosphoric/sulphuric) 125ml ACID MOLYBDATE solution 500ml ACRIDINE ORANGE (for Microscopical Staining) 10gm ACRIDINE ORANGE (for Microscopical Staining) 25gm -

Bulk Chemicals
NILE CHEMICALS CODE CAS no. ITEMS (BULK CHEMICALS) Packing A 921 64365-11-3 ACTIVATED CHARCOL 50 Drums 922 124-04-9 ADIPIC ACID 50 Drums 923 7784-26-1 ALUMINIUM AMMONIUM SULPHATE 50 Bags 11137 7446-70-0 ALUMINIUM CHLORIDE ANHYDROUS 50 Drums 924 7784-13-6 ALUMINIUM CHLORIDE HEXAHYDRATE 50 Drums 925 7784-27-2 ALUMINIUM NITRATE 50 Bags 926 10043-67-1 ALUMINIUM POTASSIUM SULPHATE 50 Bags 927 16828-11-8 ALUMINIUM SULPHATE 50 Bags 11154 12124-97-9 AMMONIUM ACETATE 50 Bags 928 3385-41-9 AMMONIUM ADIPATE 50 Drums 929 1066-33-7 AMMONIUM BI CARBONATE 50 Bags 11157 05/09/7789 AMMONIUM BI CHORMATE 50 Bags 930 12124-97-9 AMMONIUM BROMIDE 50 Drums 931 8000-73-5 AMMONIUM CARBONATE 50 Drums 11156 12125-02-9 AMMONIUM CHLORIDE 50 Bags 932 3012-65-5 AMMONIUM CITRATE DIBASIC 933 3458-72-8 AMMONIUM CITRATE TRIBASIC 934 7722-76-1 AMMONIUM DI HYDROGEN PHOSPAHTE 935 1185-57-5 AMMONIUM FERRIC CITRATE 50 Drums 936 10138-04-2 AMMONIUM FERRIC SULPHATE 50 Drums 937 7783-85-9 AMMONIUM FERROUS SULPHATE 50 Drums 938 540-69-2 AMMONIUM FORMATE 50 Bags 939 7722-76-1 AMMONIUM DIHYDROGEN PHOSPHATE 50 Bags 11158 12125-01-8 AMMONIUM FLUORIDE 11161 12054-85-2 AMMONIUM MOLYBDATE 50 Drums 940 15699-18-0 AMMONIUM NICKEL SULPHATE 50 Drums 11164 6009-70-7 AMMONIUM OXALATE 50 Bags 941 AMMONIUM PENTABORATE 50 Drums 11168 7773-06-0 AMMONIUM SULPHAMATE 50 Drums 11169 7783-20-2 AMMONIUM SULPHATE PURIFIED 50 Bags 11170 1762-95-4 AMMONIUM THIOCYANATE EXTRA PURE 50 Bags 942 1762-95-4 AMMONIUM THIOSULPHATE 943 543-80-6 BARIUM ACETATE 50 Bags 944 513-77-9 BARIUM CARBONATE 50 Bags 21114 10326-27-9 -

(12) United States Patent (10) Patent No.: US 7.462,342 B2 Tomiyama Et Al
USOO7462342B2 (12) United States Patent (10) Patent No.: US 7.462,342 B2 Tomiyama et al. (45) Date of Patent: Dec. 9, 2008 (54) METHOD FOR PRODUCING BASIC METAL (56) References Cited NITRATE U.S. PATENT DOCUMENTS (75) Inventors: Shogo Tomiyama, Hyogo (JP); Xingxi 3,983,221 A * 9, 1976 Rademachers et al. ...... 423,395 4,136,157 A * 1/1979 Asai et al. ... ... 423.395 Zhou, Hyogo (JP) 5,000,928 A * 3/1991 White ....... ... 423/34 5,039,502 A * 8/1991 Hornet al. ........ ... 423.395 (73) Assignee: Daicel Chemical Industries, Ltd., 5,429,691 A * 7/1995 Hinshaw et al. ............... 149/45 Sakai-Shi (JP) 6,468.494 B2 * 10/2002 Nappier et al. .............. 423,395 2007/01 19530 A1 5/2007 Zhou et al. *) NotOt1Ce: Subjubject to anyy d1Sclaimer,disclai theh term off thisthi patent is extended or adjusted under 35 FOREIGN PATENT DOCUMENTS U.S.C. 154(b) by 772 days. EP 1241 138 A1 9, 2002 (21) Appl. No.: 10/468,039 (Continued) (22) PCT Filed: Mar. 27, 2002 OTHER PUBLICATIONS (86). PCT No.: PCT/UP02/03005 Gmelins Handbuch Deranorganischen chemie “kuper Teil B. pp. 188-193, 1958 (corresponds to specification p. 1, line 10, no month. S371 (c)(1), (2), (4) Date: Feb. 17, 2004 (Continued) Primary Examiner Wayne Langel (87) PCT Pub. No.: WO02/079091 (74) Attorney, Agent, or Firm—Birch, Stewart, Kolasch & Birch, LLP PCT Pub. Date: Oct. 10, 2002 (57) ABSTRACT (65) Prior Publication Data US 2004/O131529 A1 Jul. 8, 2004 A process in which a basic metal nitrate is obtained in a high yield is provided.