Name: David William Grainger
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2007 PMSE Fellow Ceremony
DIVISION OF POLYMERIC MATERIALS: SCIENCE & ENGINEERING 2007 PMSE Fellow Ceremony The American Chemical Society Division of Polymeric Materials: Science and Engineering (PMSE) has just completed its process to select a new class of Fellows for 2007, and the following people have been chosen: • James Crivello • Mohamed El-Aasser • James Stoffer • Wen-Li Wu They will be inducted as the eighth class of PMSE Fellows during the PMSE/POLY Awards Reception at the Chicago ACS National Meeting on Monday, March 26, 2007. PMSE is pleased to welcome this distinguished group of polymer scientists and engineers to the ranks of fellows. A short description of their work up to the point of the induction as a PMSE Fellow is on the following pages. http://www.pmsedivision.org DIVISION OF POLYMERIC MATERIALS: SCIENCE & ENGINEERING 2007 PMSE Fellow Induction Biographies 2007 PMSE Fellow James Crivello Rensselaer Polytechnic Institute Prof. James Crivello received his B.S. in chemistry from Aquinas College in Grand Rapids, Michigan in 1962 and his Ph.D. in organic chemistry from the University of Notre Dame in 1966. He joined the General Electric Corporate Research and Development Center in 1966 and was for several years a research project manger. His fields of activity include: organic nitrations, oxidations, and arylations, polyimides, silicones, and new photo- and thermal initiators for cationic and free radical polymerizations. In 1980, he was elected a Coolidge Fellow by the staff at GE Corporate Research and Development and spent the 1986-87 year as a visiting scientist at the University of Mainz with Prof. Helmut Ringsdorf in the Federal Republic of Germany. -

Liste Des Lauréats Français Du Prix Gay-Lussac Humboldt
Liste des lauréats français du prix Gay-Lussac Humboldt >Pierre AGOSTINI (2003), Physique atomique et moléculaire, CEA* Saclay > Ohio State University Josef DEUTSCHER (2005), Microbiologie, Institut National de la Recherche Agronomique - Jacques F. ARVIEUX (1994), Physique nucléaire, Université Paris 11 CNRS, Grignon Alain ASPECT (1999), Physique, Institut d’optique, Université Paris 11 – CNRS Georges DIDI-HUBERMAN (2006), Histoire de l’art, Ecole des Hautes Etudes en Sciences Didier ASTRUC (1988), Chimie, Université Bordeaux 1 Sociales Monique AUMAILLEY (1991), Biochimie cellulaire, Université Claude Bernard - Lyon 1 > Université Pierre-Henri DIXNEUF (1989), Chimie, Université Rennes 1 de Cologne Abdelhak DJOUADI (2007), Physique des particules, Université Paris 11 Claude BARDOS (1993), Mathématiques, Université Denis Diderot - Paris 7 Jean-François DUBREMETZ (1996), Biologie, Institut national de la santé et de la recherche Jean-Marie BASSET (1987), Chimie inorganique, CNRS**, Laboratoire de chimie médicale, Villeneuve d’Ascq CNRS - Université Montpellier 2 organométallique de surface, Lyon Magda ERICSON (1992), Physique nucléaire, Université Claude Bernard - Lyon I Henri C. BENOIT (1986), Chimie macromoléculaire, Université Louis Pasteur - Strasbourg 1 Gérard FEREY (2004), Chimie, Université de Versailles - Saint-Quentin Alain BENSOUSSAN (1983), Informatique, Institut national de recherche en informatique Jean-Marie FLAUD (1998), Chimie physique, Université Paris 11 et en automatique > Centre national d’études spatiales Christos FLYTZANIS -

Ivan Julian Dmochowski, Ph.D
Ivan Julian Dmochowski, Ph.D. Department of Chemistry, University of Pennsylvania 231 South 34 th Street, Philadelphia, PA 19104-6323 P: 215-898-6459; Email: [email protected] URL: http://dmochowskigroup.chem.upenn.edu/index.html Academic Appointments Professor of Chemistry, University of Pennsylvania, July, 2015 – Present Undergraduate Chair of Chemistry, University of Pennsylvania, Jan, 2015 – Present Associate Professor of Chemistry, University of Pennsylvania, 2010 – 2015 Assistant Professor of Chemistry, University of Pennsylvania, 2003 – 2010 Education California Institute of Technology, Pasadena, CA Helen Hay Whitney Postdoctoral Scholar in Biophysics, Sept. 2000 – Dec. 2002 California Institute of Technology, Pasadena, CA Ph.D. in Chemistry, May 2000, 1995-00 Johannes Gutenberg Universität, Mainz, Germany Research Fellow in Chemistry, 1994-95 Harvard College, Cambridge, MA A.B. in Chemistry, Magna cum Laude, 1990-94 Selected Honors 2016 Crano Award, Akron Section, American Chemical Society 2012 Awardee, McKnight Technological Innovations in Neuroscience 2011 Awardee, McGroddy Frontiers in Science, St. Joseph’s Univ. 2010 Invitee, National Academies Keck Futures Initiative Imaging Meeting 2007 Camille and Henry Dreyfus Teacher-Scholar Award 2005 NSF CAREER Award 2003 Camille and Henry Dreyfus New Faculty Award 2000 Herbert Newby McCoy Award, Caltech Chemistry Department 1990 United States Presidential Scholar Fellowships 2001-02 Helen Hay Whitney Postdoctoral Fellow 1999-00 N.I.H. Bioorganic/Bioinorganic Training Grant 1996-99 N.I.H. Biotechnology Training Grant Peer-Reviewed Publications 1 1. J.A. Rego, S. Kumar, I.J. Dmochowski, H. Ringsdorf, Synthesis of novel mixed tail triphenylene discotic liquid crystals - The search for higher order . Chem. Comm. (9) 1031- 1032, 1996. -
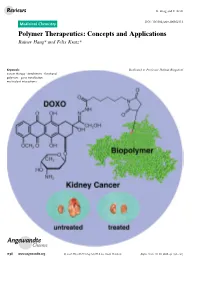
Polymer Therapeutics: Concepts and Applications Rainer Haag* and Felix Kratz*
Reviews R. Haag and F. Kratz DOI: 10.1002/anie.200502113 Medicinal Chemistry Polymer Therapeutics: Concepts and Applications Rainer Haag* and Felix Kratz* Keywords: Dedicated to Professor Helmut Ringsdorf cancer therapy · dendrimers · functional polymers · gene transfection · multivalent interactions Angewandte Chemie 1198 www.angewandte.org 2006 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Angew. Chem. Int. Ed. 2006, 45, 1198 – 1215 Angewandte Polymer Therapeutics Chemie Polymer therapeutics encompass polymer–protein conjugates, drug– From the Contents polymer conjugates, and supramolecular drug-delivery systems. Numerous polymer–protein conjugates with improved stability and 1. Introduction 1199 pharmacokinetic properties have been developed, for example, by 2. Macromolecules as Drug- anchoring enzymes or biologically relevant proteins to polyethylene Delivery Systems: Biological glycol components (PEGylation). Several polymer–protein conjugates Rationale 1201 have received market approval, for example the PEGylated form of adenosine deaminase. Coupling low-molecular-weight anticancer 3. Approaches and Applications: “In Vivo Veritas” 1203 drugs to high-molecular-weight polymers through a cleavable linker is an effective method for improving the therapeutic index of clinically 4. Summary and Conclusions 1213 established agents, and the first candidates have been evaluated in clinical trials, including, N-(2-hydroxypropyl)methacrylamide conju- gates of doxorubicin, camptothecin, paclitaxel, and platinum(ii) complexes. Another class of polymer therapeutics are drug-delivery systems based on well-defined multivalent and dendritic polymers. is covalently linked to polymers such These include polyanionic polymers for the inhibition of virus as proteins, polysaccharides, or syn- thetic polymers. attachment, polycationic complexes with DNA or RNA (polyplexes), The coupling of drugs to macro- and dendritic core–shell architectures for the encapsulation of drugs. -

Ivan Julian Dmochowski, Ph.D
Ivan Julian Dmochowski, Ph.D. Department of Chemistry, University of Pennsylvania 231 South 34 th Street, Philadelphia, PA 19104-6323 URL: http://www.sas.upenn.edu/~ivandmo/ Phone: 215-898-6459; Fax: 215-898-2037 Email: [email protected] Academic Appointments Associate Professor of Chemistry, University of Pennsylvania, 2010 – Present Assistant Professor of Chemistry, University of Pennsylvania, 2003 – 2009 Education California Institute of Technology, Pasadena, CA Helen Hay Whitney Postdoctoral Scholar in Biophysics, Sept. 2000 – Dec. 2002 California Institute of Technology, Pasadena, CA Ph.D. in Chemistry, May 2000, 1995-00 Johannes Gutenberg Universität, Mainz, Germany Research Fellow in Chemistry, 1994-95 Harvard College, Cambridge, MA A.B. in Chemistry, Magna cum Laude, 1990-94 Selected Honors 2012 Awardee, McKnight Technological Innovations in Neuroscience 2011 Awardee, McGroddy Frontiers in Science, St. Joseph’s Univ. 2010 Invitee, National Academies Keck Futures Initiative Imaging Meeting 2007 Camille and Henry Dreyfus Teacher-Scholar Award 2005 NSF CAREER Award 2003 Camille and Henry Dreyfus New Faculty Award 2000 Herbert Newby McCoy Award, Caltech Chemistry Department 1990 United States Presidential Scholar Fellowships 2001-02 Helen Hay Whitney Postdoctoral Fellow 1999-00 N.I.H. Bioorganic/Bioinorganic Training Grant 1996-99 N.I.H. Biotechnology Training Grant Peer-Reviewed Publications: h-index = 21 (March, 2013) 1. J.A. Rego, S. Kumar, I.J. Dmochowski, H. Ringsdorf, Synthesis of novel mixed tail triphenylene discotic liquid crystals - The search for higher order. Chem. Comm. (9) 1031- 1032, 1996. 1 2. J.J. Wilker, I.J. Dmochowski, J.H. Dawson, J.R. Winkler, H.B. Gray, Substrates for rapid delivery of electrons and holes to buried active sites in proteins . -

Helmut Ringsdorf
Helmut Ringsdorf Department of Organic Chemistry, University of Mainz, Duesbergweg 10-14, 55099 Mainz, Germany, Phone +49 (0)6131 3922402, Fax +49 (0)6131 3923145, e-mail: [email protected] Helmut Ringsdorf was born in Gießen/Germany in 1929. He grew up in the Rhine Valley, studied Chemistry, Politics and Geology at the Universities in Frankfurt, Darmstadt, and Freiburg. In 1955 he entered Hermann Staudingers Lab as his very last student. After his Ph.D. in Freiburg in 1958, he was a Postdoctoral Fellow with C.G. Overberger and Herman F. Mark at the Polytechnic Institute of Brooklyn (1960-1962). He taught Polymer Science and Organic Chemistry at the Universities Marburg (1962-1970) and Mainz (1971-1994). He still has the good fortune to be with young scientists as Adjunct Professor at the School of Pharmacy, University Cardiff/U.K. and at the Jilin University, Changchun/China. His awards include the H. Staudinger (Germany) as well as the H. Mark (Austria) Award, Polymer Science Award (USA). Honorary doctors from four Universities in four countries, the Friendship Award of the Chinese Government, member of Academies in Germany (Mainz, Düsseldorf), Russia (Moscow), and Italy (Catania). Based on the concept of molecular engineering of functional polymers and molecular assemblies the research interests of the group in Mainz were centred around the attempt to bridge the gap between Materials Science and Life Science as summarized in the following figure. Main research themes: Molecular architecture of functional liquid crystalline polymers, polymerization in oriented systems (micelles, multicompartment micelles, liposomes, monolayers, multilayers), properties of functional supramolecular systems, attempts to mimic biomembrane processes (enzyme functions, protein docking) and polymer therapeutics, e.g. -

Curriculum Vitae Professor Mark John Pallen
Curriculum Vitae Professor Mark John Pallen MA (Hons) Cantab, MBBS, MD, PhD January 2014 Mark Pallen — Curriculum vitae Personal Details Name Mark John Pallen Date of Birth 6 July 1960 Nationality British Address 17 Lodge Drive, Malvern Worcestershire, WR14 4LS E-mail address [email protected] Telephone 01684 567710 (home); 07824 086946 (mobile) Web Page: http://tinyurl.com/ncul2p3 Twitter: http://twitter.com/mjpallen YouTube Channel: http://www.youtube.com/user/pallenm/ Education and Qualifications PhD 1998 Imperial College, London An investigation into the links between stationary phase and virulence in Salmonella enterica enterica serovar Typhimurium MD 1993 St Bartholomew's Hospital Medical College Detection and characterisation of diphtheria toxin genes and insertion sequences MRCPath by examination in Medical Microbiology 1991 (upgraded to FRCPath 2005) MB BS 1981-84 London Hospital Medical College Undergraduate Prizes: LEPRA National Essay Prize, 1982 Turnbull Prize in Pathology, 1983, 1984 Sutton Prize in Pathology, 1983 BA (Hons) in Medical Sciences 1978-81 University of Cambridge Fitzwilliam College (Lower Second, converted to MA, 1985) Page 1 Mark Pallen — Curriculum vitae Employment Professor of Microbial Genomics Head of Division of Microbiology and Infection Apr 2013-now Warwick Medical School, University of Warwick Professor of Microbial Genomics 2001-2013 University of Birmingham Professor and Head of Department 1999-2001 Department of Microbiology and Immunobiology Queen’s University, Belfast Senior Lecturer (Honorary Consultant) 1992–99 Department of Medical Microbiology St Bartholomew's and the Royal London School of Medicine and Dentistry (Queen Mary Westfield College) Visiting Research Fellow 1994–97 Department of Biochemistry Imperial College of Science, Technology and Medicine (on a Wellcome Trust Research Leave Fellowship, working at Imperial, while still employed by Barts) Lecturer (Hon. -

LIQUID CRYSTAL NEWSLIQUID NEWS $(%(&' "!!#$(%(&'"!!# GW Gray Medal for 2006 Professor Heino Finkelmann
LIQUID CRYSTAL NEWSLIQUID NEWS $(%(&' "!!#$(%(&'"!!# GW Gray Medal for 2006 Professor Heino Finkelmann as originally intended in Krakow. Since these days, we have met very frequently, for example, when I was privileged to lecture in Professor Finkelmann’s Institute and at many other scientific meetings not least of which was the Twelfth International Liquid Crystal Conference held in 1988 in Freiburg itself and chaired and organised by Professor Finkelmann. My wife and I well remember enjoying a glass of wine with Heino and his wife and two boys at their home after the closing ceremonies were complete and people like conference chairmen are at last allowed to relax. I have put the material in the above paragraph early in this article about Heino Finkelmann, because I wanted to stress that his very great contributions to the subject of liquid crystals have been not only in the area of scientific research, but also, by his readiness to travel far and wide to Heino Finkelmann was born in Gronau in Lower give lectures, by spreading the word on the subject, by Saxony in 1945. After school, his studies at the Scientific serving on the editorial boards of relevant journals, by Technical Academy at Isny were in the field of chemical organising meetings and conferences such as the 12th engineering and on the way to qualifying in 1969, he spent ILCC, by serving as he has twice done on the Board of time with Unilever Research and with British Petroleum in Directors of the International Liquid Crystal Society and by Hamburg. He then studied Chemistry at the Technical acting as Chairman of the German Liquid Crystal Society University of Berlin, and it was here that he first began as he did for many years. -

Faculty in Pharmacotherapy
College Of Pharmacy Faculty Research Publication Revised January 2017 1 Table of Contents Faculty in Medicinal Chemistry . .03 Kuberan Balagurunathan………………………………………………………………………….………… 04 Amy M. Barrios………………………………………………………………………………………………………. 05 Grzegorz Bulaj………………………………………………………………………………………………….…. 06 Tom Cheatham III……………………………………………………………………………………………………. 07 Darrell Davis………………………………………………………………………………………………………... 08 Raphael Franzini…………………………………………………………………………………………………... 09 Margo Haygood……………………………………………………………………………………………………... 10 Chris Ireland………………………………………………………………………………………………….……... 11 Zhenjian Lin…………………………………………………………………………………………………………... 12 Shuanghu Liu…………………………………………………………………………………………………………. 13 Siam Oottamasathien..…………………………………………………………………………………………... 14 Glen Prestwich…….………………………………………………………………………………………….…… 15 Eric Schmidt….…………………………………………………………………………………………….………... 16 Robert Selliah………………………………………………………………………………………………………. 17 Jaclyn Winter………………………………………………………………………………………………………...18 Faculty in Pharmaceutics & Pharmaceutical Chemistry . .19 You Han Bae…………………………………………………………………………………………………………… 20 Mingnan chen….………………………………………………………………………………………………….…. 21 Andrew Dixon………………………………………………………………………………………………………... 22 Shuyun Dong…………………………………………………………………………………………………………. 23 Hamid Ghandehari…..……………………………………………………………………………………….……. 24 David W. Grainger……………………………………………………………………………………………….… 25 James Herron………………………………………………………………………………………………………… 26 Sung Wan Kim………………………………………………………………………………………………………… 27 Jindrich Henry Kopecek…………………………………………………………………………………….…. -

And Ph-Responsive Hydrogels and Their Controlled Release Properties
Therrno- and pH-Responsive Hydrogels and Their Controlled Release Properties Hua Yu B.S., China Textile University, China, 1984 M.S., China Textile University, China, 1987 A dissertation submitted to the faculty of the Oregon Graduate Institute of Sciences & Technology in partial fulfillment of the requirements for the degree Doctor of Philosophy in Chemistry Feburary, 1994 The dissertation "Thermo- and pH-Responsive Hydrogels and Their Controlled Release Properties" by Hua Yu has been examined and approved by the following Examination Committee: --DavidAssociatew. ProfessorGrainger:T~sis Adfisor Nini~Blackburn Professor HanS. Hoffman Professor, University of W ciences University To My Parents Acknowledgments I would like to thank my dissertation committee, Dr. David Grainger, Dr. Ninian Blackburn, Dr. Allan Hoffman, Dr. Jeffrey Hollinger, for reviewing my dissertation, special thanks to Dr. David Grainger, my thesis advisor. This research was made possible through funding from Merck Academic Predoctoral Fellowship. Discussions with Dr. Allan Hoffman, Dr. Teruo Okano, Dr. You Han Bae, and Dr. Sung-Wan Kim are gratefully appreciated. The help provided in the laboratory by Dr. Joan Sanders-Loher, Dr. Tom Loher, Dr. Jim Huntzicker and the help provided on the document by Nancy Christie, Terry Hadfield are gratefully appreciated. Also, I would like to give thanks to Jennifer Peterson and Tracey Fuller for spending their summer internship with me. TABLE OF CONTENTS Dedication i .. Acknowledgments 11 Nomenclature vii List of Figures viii List -

Cornell Chemistry November 1995 Number 64
Cornell Chemistry November 1995 Number 64 The Chairman's Notebook Science on the Silver Screen ne Friday morning last May at the American Society for Mass Spectrometry's annual meeting in Atlanta, nearly 900 scientists O dragged themselves out of bed for a plenary lecture at 8:15 a.m. Miles O'Brien, the keynote speaker, is the Cable News Network's lone science correspondent. Like many journalists who cover technological stories, O'Brien has no formal training in either mathematics or science. Once he discovered the secret to a successful story, however, those very news opportunities other reporters long ago eschewed quickly became his full-time job at CNN. His lecture was entitled 'The Geek Factor: ence that day in Atlanta, objecting to public with the excitement and passion Why Network Television Doesn't Cover O'Brien's characterization of the average only a top researcher or great teacher can Science." When conference organizers "Joe Six Pack" viewer, suggested that convey. marveled at the extraordinary turnout, CNN might have seriously underestimated O'Brien's lecture in Atlanta was full of O'Brien wryly observed that if you call its audience. Not so, O'Brien roared back. surprises. He showed videotapes of scientists by a derogatory name they will Whether they prefer cabernet, chardonnay, technology stories recently covered by always show up to find out why. Appar- Chivas, or Coors, viewers want to see CNN, some probing the underlying issues ently it goes with being an experimentalist. scientific stories on television with a clear, in depth over several minutes, which He explained that three ingredients are colorful message. -

Going with the Flow the Money Flow Issue
Drugs&DealersMagazine FEATURE: MedCity comes online Dr Eliot Forster, CEO of Creabilis and Chair of MedCity talks A Biotech and Money Publication Issue 1 | June 2014 collaboration, innovation and a new era for UK bioscience Removing the hype from IPO Building the next billion dollar businesses Horizon Discoverys’ CEO, Dr Darrin Disley talks education, Nigel Pitchford, Imperial Innovations’ CIO talks Circassia, public their high profile IPO and future growth market momentum and its own unique investment approach Tapping the markets Minding (and funding) the gap Dr Chris Blackwell, CEO of Vectura and Peter Grant, CEO of David Grainger of Index Ventures discusses their ‘market Skyepharma discuss their recent successful placements backwards’ approach to spotting innovation Taking aim at the Alternative Investment Market The Elephant in the room Lucy Tarleton of London Stock Exchange discusses IPO cycles, successful Sam Fazeli of Bloomberg and Dr Paul Cuddon of Peel Hunt floats and their new Elite programme talk market trends and dispelling the ‘B’ word Going with the Flow The money flow issue. Biotech and Money Ltd. The Euston Office | One Euston Square | 40 Melton St. | London | NW1 2FD Tel: +44 (0) 203 5744619 | Email: [email protected] Welcome A little more about Biotech and Money Biotech and Money is the first truly crowd sourced member’s only community of senior level executives from the global biopharma industry. Terence O’Dwyer The global community provides an exclusive nexus between bioscience, investors, financiers, legal and professional advisors. It’s provides a new approach to matching innovation with investors and partners. Our community helps catalyse early stage innovation through peer-to-peer education and Neil Darkes knowledge sharing, networking, partnering and Dear Reader, deal making to champion the most exciting and innovative ideas.