Scanning Electron Microscopy Cathodoluminescence of Quartz: Principles, Techniques and Applications in Ore Geology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electron Microprobe Analysis Course 12.141 Notes MIT Electron
Electron Microprobe Analysis Course 12.141 Notes Dr. Nilanjan Chatterjee The electron microprobe provides a complete micron-scale quantitative chemical analysis of inorganic solids. The method is nondestructive and utilizes characteristic x-rays excited by an electron beam incident on a flat surface of the sample. This course provides an introduction to the theory of x-ray microanalysis by wavelength and energy dispersive spectrometry (WDS and EDS), ZAF matrix correction procedures, and scanning electron imaging techniques using backscattered electron (BE), secondary electron (SE), x-ray (elemental mapping), and cathodoluminescence (CL) signals. Lab sessions involve hands- on use of the JEOL JXA-8200 Superprobe. MIT Electron Microprobe Facility Massachusetts Institute of Technology Department of Earth, Atmospheric & Planetary Sciences Room: 54-1221, Cambridge, MA 02139 Phone: (617) 253-1995; Fax: (617) 253-7102 e-mail: [email protected] web: http://web.mit.edu/e-probe/www/ Nilanjan ChatterjeeMIT, Cambridge, MA, USA October 2017 2 TABLE OF CONTENTS Page number 1. INTRODUCTION 3 2. ELECTRON SPECIMEN INTERACTIONS 5 2.1. ELASTIC SCATTERING 5 2.1.1. Electron backscattering 5 2.1.2. Electron interaction volume 6 2.2. INELASTIC SCATTERING 7 2.2.1. Secondary electron generation 7 2.2.2. Characteristic x-ray generation: inner-shell ionization 7 2.2.3. X-ray production volume 10 2.2.4. Bremsstrahlung or continuum x-ray generation 11 2.2.5. Cathodoluminescence 12 3. QUANTITATIVE X-RAY SPECTROMETRY 13 3.1. MATRIX CORRECTIONS 14 3.1.1. Atomic number correction (Z) 14 3.1.2. Absorption correction (A) 16 3.1.3. -

Electron Probe Microanalysis (EPMA) in the Earth Sciences
This is a repository copy of Electron probe microanalysis (EPMA) in the Earth Sciences. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/135874/ Version: Accepted Version Proceedings Paper: Walshaw, R orcid.org/0000-0002-8319-9312 (Accepted: 2018) Electron probe microanalysis (EPMA) in the Earth Sciences. In: Book of Tutorials and Abstracts. Microbeam Analysis in the Earth Sciences, 13th EMAS Regional Workshop, 04-07 Sep 2018, University of Bristol. (In Press) Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ ELECTRON PROBE MICROANALYSIS (EPMA) IN THE EARTH SCIENCES R.D. Walshaw University of Leeds, School of Earth & Environment, Leeds Electron Microscopy & Spectroscopy Centre Woodhouse Lane, Leeds LS2 9JT, Great Britain e-mail: [email protected] 75 1. INTRODUCTION Electron probe microanalysis (EPMA) is a non-destructive, standards-based microanalytical technique, yielding fully quantitative chemical analyses of solid materials at the micron scale. -

Cathodoluminescence in Semiconductor Structures Under Local Tunneling Electron Injection Petr Polovodov
Cathodoluminescence in semiconductor structures under local tunneling electron injection Petr Polovodov To cite this version: Petr Polovodov. Cathodoluminescence in semiconductor structures under local tunneling electron injection. Optics [physics.optics]. Ecole Doctorale de l’Ecole Polytechnique, 2015. English. tel- 01346834v2 HAL Id: tel-01346834 https://hal.archives-ouvertes.fr/tel-01346834v2 Submitted on 23 Jul 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Thèse présentée pour obtenir le grade de DOCTEUR DE L'ÉCOLE POLYTECHNIQUE Spécialité : Physique Par Petr POLOVODOV Cathodoluminescence in semiconductor structures under local tunneling electron injection Soutenue le 21 décembre 2015 devant le jury composé de : M. Fabrice Charra CEA/Saclay, Gif sur Yvette Rapporteur M. Philippe Dumas CINaM, Marseille Rapporteur M. Razvigor Ossikovski LPICM, Ecole Polytechnique, Palaiseau Examinateur M. Oleg Tereshchenko Rzhanov Institute of Semiconductor Examinateur Physics, Novosibirsk, Russie M. Yves Lassailly LPMC, Ecole Polytechnique, Palaiseau Directeur de thèse M. Jacques Peretti LPMC, Ecole Polytechnique, Palaiseau Co-Directeur de thèse 2 ACNOWLEGEMENTS I have defended my PhD thesis in physics. This is the accomplishment of myself but several persons have contributed to this work. First of all, I am grateful to François Ozanam and Mathis Plapp, successive Directors of the Laboratory, who allowed me to perform my PhD work at the LPMC in the best possible conditions. -

Low-Voltage Cathodoluminescent Phosphors a 20-Year Chronology of Low-Voltage Cathodoluminescence Efficiency by Lauren E
Low-Voltage Cathodoluminescent Phosphors A 20-year chronology of low-voltage cathodoluminescence efficiency by Lauren E. Shea hosphors have been used for the display of information since the invention of the cathode-ray tube (CRT) by Karl Ferdinand Braun in 1897 (1). With the P development of color television, an effort spanning approximately thirty years, came the most significant advances in phosphor technology. The most noteworthy was the shift to the all-sulfide system, and discovery of the red, rare-earth oxysulfide phosphors 3+ (e.g., Y2O2S:Eu ) (2). White brightness efficiency of phosphor screens also improved significantly: 15 lm/W in FIG. 1. Efficiency of red phosphor powders (lettered squares) and screens (numbered diamonds) as a function of electron accelerating voltage. Y O :Eu - A, I, S (12); G (13); N (20); O, U, 5, 9 (14); W (21); YVO :Eu - B, L, 1951 to over 35 lm/W in 1979, as a 2 3 4 Q (13); F (18); P, X, 7, 10 (14); Y2O2S:Eu - C, J, R (22); D(12); H, Y (20); K, V (21); M, T, 3, 6, 11 (14); 8 result of new phosphor formulations (23); ZnCdS:Ag, In - 1 (16); ZnCdS:Ag, In + SnO2 - 2 (17); LaInO3:Eu - 4 (15); unspecified - E (18). and improved screening techniques (3). Today, a primary focus of research toire d’Electronique de Technologie et reported in earlier years, and (4) has in the area of luminescence is phosphor d’Instrumentation (LETI) (5). Many progress been made? development and improvement for FEDs are being designed for operation in ≤ low-voltage ( 1 kV) emissive flat-panel the 5-10 kV range. -

The Chemical Activators of Cathodoluminescence in Jadeite
The Chemical Activators of Cathodoluminescence in Jadeite Erin C. Dopfel A thesis presented to the faculty of Mount Holyoke College in partial fulfillment of the requirements for the degree of Bachelor of Arts with Honor Department of Earth and Environment Mount Holyoke College South Hadley, Massachusetts May 2006 Thesis Advisor: Dr. M. Darby Dyar Department Chair: Dr. Mark McMenamin ACKNOWLEDGMENTS I would like to thank my advisor, Darby Dyar, for her patience and guidance, and all the support she has shown toward this project over the past year. I am also grateful to Dean Connie Allen and Mark McMenamin, members of my thesis committee, for reading this document and providing me with extraordinarily helpful suggestions. And to the faculty of Mount Holyoke’s Department of Earth and Environment, especially Michelle Markley, Al Werner, and Steve Dunn, who have acted not only as mentors, but as true friends. Endless thanks to the donors of the Mount Holyoke Summer Research Fellowship, the Martha Godchaux Field Scholarship, and the Department of Earth and Environment funds, as well as Darby Dyar and the Sorena Sorensen, for supporting my travels and research expenses. Thanks to Mike Jercinovic at the University of Massachusetts – Amherst Microprobe Lab, who somehow managed to run all of my EPMA analyses on the shortest-notice-known-to-man. I am especially grateful to William F. McDonough and Richard Ash at the University of Maryland’s Isotope Geochemistry Lab, for allowing me to utilize their facilities to collect my LA ICP-MS data. Also, thanks to Gerard Marchand, Yarrow Rothstein, and Eli Sklute of Mount Holyoke College for helping to prepare and change ii samples, and fit messy Mössbauer curves. -

Multi-Color Correlative Light and Electron Microscopy Using Nanoparticle Cathodoluminescence
Multi-color correlative light and electron microscopy using nanoparticle cathodoluminescence D.R. Glenn1, H. Zhang1, N. Kasthuri2,3, R. Schalek2,3, P.K. Lo4, A.S. Trifonov1, H. Park4, J.W. Lichtman2,3, R.L. Walsworth1,3,5 1.Harvard-Smithsonian Center for Astrophysics, Cambridge, MA 02138, 2.Department of Molecular and Cellular Biology, 3.Center for Brain Science, 4.Department of Chemistry, 5.Department of Physics, Harvard University, Cambridge, MA 02138 Abstract: Correlative light and electron microscopy promises to combine molecular specificity with nanoscale imaging resolution. However, there are substantial technical challenges including reliable co-registration of optical and electron images, and rapid optical signal degradation under electron beam irradiation. Here, we introduce a new approach to solve these problems: multi-color imaging of stable optical cathodoluminescence emitted in a scanning electron microscope by nanoparticles with controllable surface chemistry. We demonstrate well-correlated cathodoluminescence and secondary electron images using three species of semiconductor nanoparticles that contain defects providing stable, spectrally-distinguishable cathodoluminescence. We also demonstrate reliable surface functionalization of the particles. The results pave the way for the use of such nanoparticles for targeted labeling of surfaces to provide nanoscale mapping of molecular composition, indicated by cathodoluminescence color, simultaneously acquired with structural electron images in a single instrument. Introduction: The correlation of light microscopy with electron microscopy offers considerable scope for new discovery and applications in the physical and life sciences by providing images with both molecular specificity and nanoscale spatial resolution [1]. However, such an approach also faces substantial technical challenges in the reliable and efficient co-registration of optical and electron images [2] [3] [4]. -

Scanning Electron Microscopy, Cathodoluminescence, and Raman Spectroscopy of Experimentally Shock-Metamorphosed Quartzite
Meteoritics & Planetary Science 38, Nr 8, 1187–1197 (2003) Abstract available online at http://meteoritics.org Scanning electron microscopy, cathodoluminescence, and Raman spectroscopy of experimentally shock-metamorphosed quartzite Arnold GUCSIK, 1 Christian KOEBERL, 1* Franz BRANDSTÄTTER, 2 Eugen LIBOWITZKY, 3 and Wolf Uwe REIMOLD 4 1Department of Geological Sciences, University of Vienna, Althanstrasse 14, A-1090 Vienna, Austria 2Department of Mineralogy, Natural History Museum, P.O. Box 417, A-1014 Vienna, Austria 3Institute of Mineralogy and Crystallography, University of Vienna, Althanstrasse 14, A-1090 Vienna, Austria 4Impact Cratering Research Group, School of Geosciences, University of Witwatersrand, Private Bag 3, P.O. 2050, Johannesburg, South Africa *Corresponding author. E-mail: [email protected] (Received 30 December 2002; revision accepted 8 July 2003) Abstract–We studied unshocked and experimentally (at 12, 25, and 28 GPa, with 25, 100, 450, and 750°C pre-shock temperatures) shock-metamorphosed Hospital Hill quartzite from South Africa using cathodoluminescence (CL) images and spectroscopy and Raman spectroscopy to document systematic pressure or temperature-related effects that could be used in shock barometry. In general, CL images of all samples show CL-bright luminescent patchy areas and bands in otherwise non- luminescent quartz, as well as CL-dark irregular fractures. Fluid inclusions appear dominant in CL images of the 25 GPa sample shocked at 750°C and of the 28 GPa sample shocked at 450°C. Only the optical image of our 28 GPa sample shocked at 25°C exhibits distinct planar deformation features (PDFs). Cathodoluminescence spectra of unshocked and experimentally shocked samples show broad bands in the near-ultraviolet range and the visible light range at all shock stages, indicating the presence of defect centers on, e.g., SiO 4 groups. -

Photoluminescence and Cathodoluminescence Properties of a Novel 3+ Calaga3o7:Dy Phosphor
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Article Materials Science March 2012 Vol.57 No.7: 827831 doi: 10.1007/s11434-011-4938-5 Photoluminescence and cathodoluminescence properties of a novel 3+ CaLaGa3O7:Dy phosphor ZHAO WenYu1,2*, AN ShengLi1, FAN Bin1, LI SongBo1 & DAI YaTang3 1 School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, Beijing 100083, China; 2 School of Chemistry and Chemical Engineering, Inner Mongolia University of Science and Technology, Baotou 014010, China; 3 School of Materials Science and Engineering, Southwest University of Science and Technology, Mianyang 621000, China Received July 14, 2011; accepted October 19, 2011; published online December 27, 2011 3+ A single host white emitting phosphor, CaLaGa3O7:Dy , was synthesized by chemical co-precipitation. Field emission scanning electron microscopy, X-ray diffraction, laser particle size analysis, and photoluminescence and cathodoluminescence spectra were used to investigate the structural and optical properties of the phosphor. The phosphor particles were composed of microspheres with a slight tendency to agglomerate, and an average diameter was of about 1.0 m. The Dy3+ ions acted as luminescent centers, 3+ and substituted La ions in the single crystal lattice of CaLaGa3O7 where they were located in Cs sites. Under excitation with 3+ 3+ ultraviolet light and a low voltage electron beam, the CaLaGa3O7:Dy phosphor exhibited the characteristic emission of Dy 4 6 4 6 ( F9/2- H15/2 and F9/2- H13/2 transitions) with intense yellow emission at about 573 nm. The chromaticity coordinates for the phos- phor were in the white region. -

Application of Cathodoluminescence in Paint Analysis
Scanning Microscopy Volume 6 Number 3 Article 4 6-30-1992 Application of Cathodoluminescence in Paint Analysis W. Stoecklein Bundeskriminalamt R. Göbel Bundeskriminalamt Follow this and additional works at: https://digitalcommons.usu.edu/microscopy Part of the Biology Commons Recommended Citation Stoecklein, W. and Göbel, R. (1992) "Application of Cathodoluminescence in Paint Analysis," Scanning Microscopy: Vol. 6 : No. 3 , Article 4. Available at: https://digitalcommons.usu.edu/microscopy/vol6/iss3/4 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Scanning Microscopy by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Scanning Microscopy, Vol. 6, No. 2, 1992 (Pages 669-678) 0891- 7035/92$5. 00 +. 00 Scanning Microscopy International, Chicago (AMF O'Hare), IL 60666 USA APPLICATION OF CATHODOLUMINESCENCE IN PAINT ANALYSIS W. Stoecklein* and R. Gobel Bundeskriminalamt, Forensic Science Institute D-6200 Wiesbaden, Federal Republic of Germany (Received for publication May 3, 19921, and in revised form June 30, 1992) Abstract Introduction When solving cases of burglary or investigating Paints and their components often play a decisive ship collisions, the forensic scientist frequently has to role as evidence in solving crimes. The forensic scien examine several layers of paint of the same color, often tist analyzing such material must not only determine the white. As a rule, the usual microscopic and spectro origin of unknown paints (identification and classifica scopic methods [fluorescence microscopy, FT-IR (Four tion) but in addition compare traces collected from ob ier Transform Infrared Spectroscopy), pyrolysis, GC/MS jects in the suspect's possession or found in his environ (Gas Chromatography - Mass Spectrometry), etc.] are ment with those traces secured at the scene of the crime. -
Electron Microprobe Analysis Slide 4
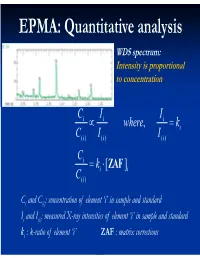
EPMA: Quantitative analysis WDS spectrum: Intensity is proportional to concentration C i I i Ii where, ki C( i )I i ( ) I() i C i ki [ ZAF i ] C( i ) Ci and C(i): concentration of element ‘i’ in sample and standard Ii and I(i): measured X-ray intensities of element ‘i’ in sample and standard ki : k-ratio of element ‘i’ ZAF : matrix corrections Matrix (ZAF) corrections Z : atomic number correction A : absorption correction F : fluorescence correction Atomic number (Z) correction R = CjRj Ri [ R = #X-ray photons S i generated / #photons if Z i * there were no back-scatter] Ri * S = C S S i j j [ S = -(1/)(dE/ds), * sample stopping power] Z, a function of E0 and composition Duncumb-Reed-Yakowitz method: Ri = j CjRij Rij = R' 1 - R'2 ln(R'3 Zj+25) -3 3 2 R'1 = 8.73x10 U - 0.1669 U + 0.9662 U + 0.4523 -3 3 -2 2 R'2 = 2.703x10 U - 5.182x10 U + 0.302 U - 0.1836 3 2 3 R'3 = (0.887 U - 3.44 U + 9.33 U - 6.43)/U Si = j CjSij Sij = (const) [(2Zj/Aj )/(E0+Ec)]ln[583(E0+Ec)/Jj ] where, J (keV) = (9.76Z + 58.82Z-0.19)x10-3 Z, a function of E0 and composition Al-Cu alloy 1.2 1.2 Pure metal standards Pure metal standards 1.15 1.15 1.1 1.1 1.05 1.05 1 1 AlK C CuK C 0.95 Cu 0.95 Cu Z 0.1 0.1 Z 0.9 0.3 0.9 0.3 0.5 0.5 0.85 0.7 0.85 0.7 0.9 0.9 0.8 0.8 10 15 20 10 15 20 E0 (keV) E0 (keV) 1.2 1.2 CuAl standard CuAl standard 1.15 2 1.15 2 1.1 1.1 1.05 1.05 1 1 AlK C CuK C 0.95 Cu 0.95 Cu Z 0.1 0.1 Z 0.9 0.3 0.9 0.3 0.5 0.5 0.85 0.7 0.85 0.7 0.9 0.9 0.8 0.8 10 15 20 10 15 20 E0 (keV) E0 (keV) X-ray absorption -(/)(x) -(/)( z cosec) I = I0 exp = I0 -

Dislocation Related Droop in Ingan/Gan Light Emitting Diodes Investigated Via Cathodoluminescence
Dislocation related droop in InGaN/GaN light emitting diodes investigated via cathodoluminescence Galia Pozina, Rafal Ciechonski, Zhaoxia Bi, Lars Samuelson and Bo Monemar Linköping University Post Print N.B.: When citing this work, cite the original article. Original Publication: Galia Pozina, Rafal Ciechonski, Zhaoxia Bi, Lars Samuelson and Bo Monemar, Dislocation related droop in InGaN/GaN light emitting diodes investigated via cathodoluminescence, 2015, Applied Physics Letters, (107), 25, 251106. http://dx.doi.org/10.1063/1.4938208 Copyright: American Institute of Physics (AIP) http://www.aip.org/ Postprint available at: Linköping University Electronic Press http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-125162 Dislocation related droop in InGaN/GaN light emitting diodes investigated via cathodoluminescence Galia Pozina, Rafal Ciechonski, Zhaoxia Bi, Lars Samuelson, and Bo Monemar Citation: Applied Physics Letters 107, 251106 (2015); doi: 10.1063/1.4938208 View online: http://dx.doi.org/10.1063/1.4938208 View Table of Contents: http://scitation.aip.org/content/aip/journal/apl/107/25?ver=pdfcov Published by the AIP Publishing Articles you may be interested in High-power low-droop violet semipolar ( 30 3 ¯ 1 ¯ ) InGaN/GaN light-emitting diodes with thick active layer design Appl. Phys. Lett. 105, 171106 (2014); 10.1063/1.4900793 Influence of dislocation density on carrier injection in InGaN/GaN light-emitting diodes operated with alternating current Appl. Phys. Lett. 102, 011115 (2013); 10.1063/1.4773588 Dependence of radiative efficiency and deep level defect incorporation on threading dislocation density for InGaN/GaN light emitting diodes Appl. Phys. Lett. 101, 162102 (2012); 10.1063/1.4759003 Improvements of external quantum efficiency of InGaN-based blue light-emitting diodes at high current density using GaN substrates J. -

ANL-7917 Chemical Separations Processes for Plutonium and Uranium
ANL-7917 Chemical Separations Processes for Plutonium and Uranium ARGONNE NATIONAL LABORATORY 9700 South Cass Avenue Argonne, Illinois 60439 DEVELOPMENT STUDIES ON A FLUIDIZED-BED PROCESS FOR CONVERSION OF U/Pu NITRATES TO OXIDES Part 1. Laboratory-scale Denitration Studies by S. Vogler, D. E. Grosvenor, N. M. Levitz, and F. G. Teats Chemical Engineering Division April 1972 NOTICE- This report was prepared as an account of work sponsored by the United States Government. Neither the United States nor the United States Atomic Energy Commission, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, com• pleteness or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. BOTBimON DF THIS DOCUMENT IS ONLI DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency Thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.