PATIENT INFORMATION LEAFLET KENALOG™ INTRA-ARTICULAR / INTRAMUSCULAR INJECTION 40 Mg/Ml Triamcinolone Acetonide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Fact Sheet Provides Information to Patients with Eczema and Their Carers. About Topical Corticosteroids How to Apply Topic
This fact sheet provides information to patients with eczema and their carers. About topical corticosteroids You or your child’s doctor has prescribed a topical corticosteroid for the treatment of eczema. For treating eczema, corticosteroids are usually prepared in a cream or ointment and are applied topically (directly onto the skin). Topical corticosteroids work by reducing inflammation and helping to control an over-reactive response of the immune system at the site of eczema. They also tighten blood vessels, making less blood flow to the surface of the skin. Together, these effects help to manage the symptoms of eczema. There is a range of steroids that can be used to treat eczema, each with different strengths (potencies). On the next page, the potencies of some common steroids are shown, as well as the concentration that they are usually used in cream or ointment preparations. Using a moisturiser along with a steroid cream does not reduce the effect of the steroid. There are many misconceptions about the side effects of topical corticosteroids. However these treatments are very safe and patients are encouraged to follow the treatment regimen as advised by their doctor. How to apply topical corticosteroids How often should I apply? How much should I apply? Apply 1–2 times each day to the affected area Enough cream should be used so that the of skin according to your doctor’s instructions. entire affected area is covered. The cream can then be rubbed or massaged into the Once the steroid cream has been applied, inflamed skin. moisturisers can be used straight away if needed. -
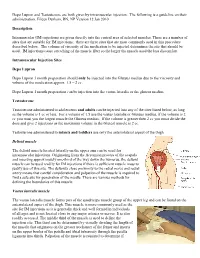
An Intramuscular Injection Is an Injection Given Directly Into The
Depo Lupron and Testosterone are both given by intramuscular injection. The following is a guideline on their administration. Eileen Durham, RN, NP Version 12 Jan 2010 Description Intramuscular (IM) injections are given directly into the central area of selected muscles. There are a number of sites that are suitable for IM injections; there are three sites that are most commonly used in this procedure described below. The volume of viscosity of the medication to be injected determines the site that should be used. IM injections cause stretching of the muscle fiber so the larger the muscle used the less discomfort. Intramuscular Injection Sites Depo Lupron Depo Lupron 3 month preparation should only be injected into the Gluteus medius due to the viscosity and volume of the medication approx. 1.5 – 2 cc. Depo Lupron 1 month preparation can be injection into the vastus lateralis or the gluteus medius. Testosterone Testosterone administered to adolescents and adults can be injected into any of the sites listed below, as long as the volume is 1 cc or less. For a volume of 1.5 use the vastus lateralis or Gluteus medius, if the volume is 2 cc you must you the largest muscle the Gluteus medius. If the volume is greater then 2 cc you must divide the dose and give 2 injections as the maximum volume in the Gluteal muscle is 2 cc. Testosterone administered to infants and toddlers use only the anteriolateral aspect of the thigh. Deltoid muscle The deltoid muscle located laterally on the upper arm can be used for intramuscular injections. -

Determination of Triamcinolone Acetonide Steroid on Glassy Carbon Electrode by Stripping Voltammetric Methods
Int. J. Electrochem. Sci., 3 (2008) 509-518 International Journal of ELECTROCHEM ICAL SCIENCE www.electrochemsci.org Determination of Triamcinolone Acetonide Steroid on Glassy Carbon Electrode by Stripping Voltammetric Methods C. Vedhi, R. Eswar, H. Gurumallesh Prabu and P. Manisankar* Department of Industrial Chemistry, Alagappa University, Karaikudi –630 003,Tamil Nadu, India *E-mail: [email protected] or [email protected] Received: 9 December 2007 / Accepted: 6 February 2008 / Online published: 20 February 2008 A corticosteroid, triamcinolone acetonide was determined by stripping voltammetric procedure using glassy carbon electrode. Cyclic voltammetric behaviour of steroid was studied in 50% aqueous methanol at acid, neutral and alkaline pH conditions. Influence of pH, sweep rate and steroid concentration were studied. An irreversible and adsorption-controlled well-defined reduction peak was observed in all pH conditions. The reduction peak potential shifted cathodically with change in pH. Controlled potential coulometry revealed two-electron reduction of the α,β-unsaturated carbonyl function present in the steroid. A systematic study of the experimental parameters that affect the differential pulse / square wave stripping voltammetric response was carried out and optimized conditions were arrived at. Under optimum conditions, differential pulse and square wave adsorptive stripping voltammetric procedures were developed for the determination of triamcinolone acetonide steroid at pH 13.0. A calibration plots was derived and the lower limit of determination observed are 0.1 µg/mL from DPSV and 0.01 µg/mL from SWSV. Keywords: Triamcinolone acetonide steroid, Cyclic Voltammetry, Stripping Voltammetry, Glassy carbon electrode. 1. INTRODUCTION Corticosteroids are hormones produced naturally by the adrenal glands, which have many important functions on every organ system. -

Injection Technique 1: Administering Drugs Via the Intramuscular Route
Copyright EMAP Publishing 2018 This article is not for distribution except for journal club use Clinical Practice Keywords Intramuscular injection/ Medicine administration/Absorption Practical procedures This article has been Injection technique double-blind peer reviewed Injection technique 1: administering drugs via the intramuscular route rugs administered by the intra- concerns that nurses are still performing Author Eileen Shepherd is clinical editor muscular (IM) route are depos- outdated and ritualistic practice relating to at Nursing Times. ited into vascular muscle site selection, aspirating back on the syringe Dtissue, which allows for rapid (Greenway, 2014) and skin cleansing. Abstract The intramuscular route allows absorption into the circulation (Dough- for rapid absorption of drugs into the erty and Lister, 2015; Ogston-Tuck, 2014). Site selection circulation. Using the correct injection Complications of poorly performed IM Four muscle sites are recommended for IM technique and selecting the correct site injection include: administration: will minimise the risk of complications. l Pain – strategies to reduce this are l Vastus lateris; outlined in Box 1; l Rectus femoris Citation Shepherd E (2018) Injection l Bleeding; l Deltoid; technique 1: administering drugs via l Abscess formation; l Ventrogluteal (Fig 1, Table 1). the intramuscular route. Nursing Times l Cellulitis; Traditionally the dorsogluteal (DG) [online]; 114: 8, 23-25. l Muscle fibrosis; muscle was used for IM injections but this l Injuries to nerves and blood vessels muscle is in close proximity to a major (Small, 2004); blood vessel and nerves, with sciatic nerve l Inadvertent intravenous (IV) access. injury a recognised complication (Small, These complications can be avoided if 2004). -

Self-Administering a Vitamin B12 Injection
Self-administering a Vitamin B12 injection This is usually given as an intramuscular injection, every 2-3 months. Alternatives to an intramuscular injection are: Oral Vitamin B12 at a dose of at least 1000mcg per day. • Available as tablets or a spray. They can be bought over the counter and available at most health food stores and pharmacies. • Oral Vitamin B12 is not recommended if: - If you have a bowel condition such as inflammatory bowel disease, Coeliac disease, small bowel overgrowth, bile acid malabsorption and short bowel (you will require injections) - You require an initial loading of B12 (soon after diagnosis) It is important to monitor your symptoms if you change to oral B12. If symptoms return, then the oral/sublingual dose can be increased to 2000mcg or you may need to consider starting back on injections. https://www.hollandandbarrett.com/shop/product/betteryou-pure-energy-b12-boost-oral-spray-60099160?skuid=099160 https://www.hollandandbarrett.com/search?query=%20Vitamin%20B12%20Tablets&utm_medium=cpc&utm_source=google&isSearch=true# gclid=EAIaIQobChMIh5nLgOH26AIVxLTtCh0JIA_GEAAYASAAEgJL-fD_BwE&gclsrc=aw.ds Subcutaneous (SC) injection; this is off-licence but still effective. It is considered an easier method of administration. It is how insulin and blood thinning medication are usually self-administered. Equipment needed to self-inject - Prescribed medicine - 1 syringe (2 ml) - 2 needles (1 for drawing up the drug and 1 for administration – you can use the same size needle for both). o For an IM injection; the needle gauge should be 19-25. The needle length is 1- 1 ½ inches (up to 3 inches for larger adults) o For a SC injection; the needle gauge should be 25-27. -

Canine Vascular Tissues Are Targets for Androgens, Estrogens, Progestins, and Glucocorticoids
Canine vascular tissues are targets for androgens, estrogens, progestins, and glucocorticoids. K B Horwitz, L D Horwitz J Clin Invest. 1982;69(4):750-758. https://doi.org/10.1172/JCI110513. Research Article Sex differences and steroid hormones are known to influence the vascular system as shown by the different incidence of atherosclerosis in men and premenopausal women, or by the increased risk of cardiovascular diseases in women taking birth control pills or men taking estrogens. However, the mechanisms for these effects in vascular tissues are not known. Since steroid actions in target tissues are mediated by receptors, we have looked for cytoplasmic steroid receptor proteins in vascular tissues of dogs. We find specific saturable receptors, sedimenting at 8S on sucrose density gradients for estrogens (measured with [3H]estradiol +/- unlabeled diethylstilbestrol), androgens (measured with [3H]R1881 +/- unlabeled R1881 and triamcinolone acetonide), and glucocorticoids (measured with [3H]dexamethasone +/- unlabeled dexamethasone); they are absent for progesterone (measured with [3H]R5020 +/- unlabeled R5020 and dihydrotestosterone). Progesterone receptors can, however, be induced by 1-wk treatment of dogs with physiological estradiol concentrations (100 pg/ml serum estrogen), indicating a functional estrogen receptor. Receptor levels range from 20 to 2,000 fmol/mg DNA. They are specific for each hormone; unrelated steroids fail to complete for binding. Low dissociation constants, measured by Scatchard analyses, show that binding is of high affinity. Steroid binding sites are in the media and/or adventitia since they persist when the intima is removed. Compared with the arteries, receptor levels are reduced 80% in inferior venae cavae of […] Find the latest version: https://jci.me/110513/pdf Canine Vascular Tissues Are Targets for Androgens, Estrogens, Progestins, and Glucocorticoids KATHRYN B. -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

Guidelines for Management of Acne
GUIDELINES FOR MANAGEMENT OF ACNE PAGE 1. Classification of Acne 2 2. Medication available at ANMC 2 3. Adjunctive Measures 3 4. Acne treatment by severity or type 3 5. Patient Education 4 Revised by : Jennifer Stroh x3317 Revision Date: 03/01/06 PIC Approval Date: 09/07/06 1 TREATMENT OF ACNE Classification of Acne Severity Papules/Pustules Nodules Comedonal None to few None Mild Few to several None Moderate Several to many Few to several Severe Numerous or extensive Many Medications available at ANMC for acne treatment Topical 1. Bacteriocidal: - Benzoyl peroxide (OTC-non formulary) - antimicrobicidal gel, cream, lotion (2.5, 5, 10%). Apply qhs or bid. May irritate skin resulting in redness and scaling which usually resolves with continued use. 2. Topical antibiotics: - Clindamycin solution. Apply bid - Benzamycin gel* (Erythromycin 3%, Benzoyl peroxide 5%). Apply qhs initially, advance to bid if indicated * Benzamycin gel is second line (restricted per ANMC’s formulary to failed topical clindamycin). 3. Retinoids: - Tretinoin (Retin-A) cream 0.025%, 0.1%. Start with lower strength, applied 2-3 times/wk. Wash skin before bedtime and allow to dry for 30 mins before applying tretinoin. Redness and scaling may occur and acne may worsen over 2-4 weeks. Benzoyl peroxide oxidizes tretinoin, so don’t apply at the same time. Systemic 1. Oral antibiotics. Start with high dose and gradually taper over 2-4 months to lowest maintenance dose required to maintain control. Instruct patients to increase dose with first sign of flare-up. - Tetracycline-250mg, 500mg. Start with 500mg bid, taper monthly to 250 mg bid, then qd or qod. -

Efficacy/Risk Profile of Triamcinolone Acetonide in Severe Asthma
ARTICLE IN PRESS Respiratory Medicine CME (2008) 1, 111–115 respiratory MEDICINE CME CASE REPORT Efficacy/risk profile of triamcinolone acetonide in severe asthma: Lessons from one case study Cesar Picadoà Servei de Pneumologia, Hospital Clinic, Universitat de Barcelona, Villarroel 170, 08036 Barcelona, Spain Received 26 November 2007; accepted 22 January 2008 KEYWORDS Summary Insulin; Some prednisone-resistant asthma patients respond to intramuscular triamcinolone Refractory asthma; acetonide (TA). The use of TA has been questioned on the basis of its potential toxicity. Severe asthma; TA’s ratio of clinical efficacy to systemic effects compared with oral prednisone has not Steroid-dependent been clearly defined. asthma; We report the case of a prednisone-insensitive severe asthma patient with insulin- Triamcinolone dependent diabetes, hypertension and diabetic retinopathy treated with repeated sessions acetonide of laser therapy. By daily monitoring the needs of insulin doses we compared the systemic effects of prednisone and TA. The analysis of the balance between systemic effects (insulin doses) and beneficial effects (PEF values) show that TA has a better benefit/risk profile than prednisone. The patient has been followed up for 3 years and has received injections of TA repeated at intervals ranging from 21 to 60 days according to patient response and the evolution of PEF and clinical symptoms. During this period the patient lost 3 kg of weight, while presenting increased bone mineral density, normalized arterial tension, and improved proliferative diabetic retinopathy. The present case report shows that TA has a better benefit/risk profile than prednisone. TA can be a valuable alternative in the control of some patients with severe prednisone- resistant asthma. -

How to Administer Intramuscular, Intradermal, and Intranasal Influenza Vaccines
How to Administer Intramuscular, Intradermal, and Intranasal Influenza Vaccines Intramuscular injection (IM) Intradermal administration (ID) Intranasal administration (NAS) Inactivated Influenza Vaccines (IIV), including Inactivated Influenza Vaccine (IIV) Live Attenuated Influenza Vaccine (LAIV) recombinant hemagglutinin influenza vaccine (RIV3) 1 Gently shake the microinjection system before 1 FluMist (LAIV) is for intranasal administration 1 Use a needle long enough to reach deep into administering the vaccine. only. Do not inject FluMist. the muscle. Infants age 6 through 11 mos: 1"; 1 through 2 yrs: 1–1¼"; children and adults 2 Hold the system by placing the 2 Remove rubber tip protector. Do not remove 3 yrs and older: 1–1½". thumb and middle finger on dose-divider clip at the other end of the sprayer. the finger pads; the index finger 2 With your left hand*, bunch up the muscle. should remain free. 3 With the patient in an upright position, place the tip just inside the nostril 3 With your right hand*, insert the needle at a 3 Insert the needle perpendicular to the skin, to ensure LAIV is delivered into 90° angle to the skin with a quick thrust. in the region of the deltoid, in a short, quick the nose. The patient should movement. breathe normally. 4 Push down on the plunger and inject the entire contents of the syringe. There is no need to 4 Once the needle has been 4 With a single motion, depress plunger as aspirate. inserted, maintain light pressure rapidly as possible until the dose-divider clip on the surface of the skin and prevents you from going further. -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -

Utah Medicaid Pharmacy and Therapeutics Committee Drug
Utah Medicaid Pharmacy and Therapeutics Committee Drug Class Review Single Ingredient Nasal Corticosteroids Beclomethasone dipropionate (Qnasl) Beclomethasone dipropionate monohydrate (Beconase AQ) Budesonide (Rhinocort) Ciclesonide (Omnaris, Zetonna) Flunisolide (Generic) Fluticasone Furoate (Flonase Sensimist) Fluticasone Propionate (Flonase, Xhance) Mometasone Furoate (Nasonex) Triamcinolone Acetonide (Nasacort) AHFS Classification: 52.08.08 Corticosteroids (EENT) Final Report February 2018 Review prepared by: Valerie Gonzales, Pharm.D., Clinical Pharmacist Elena Martinez Alonso, B.Pharm., MSc MTSI, Medical Writer Vicki Frydrych, Pharm.D., Clinical Pharmacist Joanita Lake, B.Pharm., MSc EBHC (Oxon), Research Assistant Professor Joanne LaFleur, Pharm.D., MSPH, Associate Professor University of Utah College of Pharmacy University of Utah College of Pharmacy, Drug Regimen Review Center Copyright © 2018 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved Contents Abbreviations ................................................................................................................................................ 2 Executive Summary ....................................................................................................................................... 3 Introduction .................................................................................................................................................. 5 Table 1. Nasal corticosteroid products .............................................................................................